Clinical Study vs. Clinical Evaluation: An Overview
If you’re in the medical device industry, you’ve probably come across the terms Clinical Study and Clinical Evaluation more than once. While they might seem similar, they serve different purposes and are used in different situations. Understanding these differences is crucial, especially when navigating regulatory requirements for market approval.
So, let’s break it down in a simple and practical way—no heavy jargon, just the essential information you need!
Looking For a Medical Device Regulatory Consultant?
What is Clinical Study?
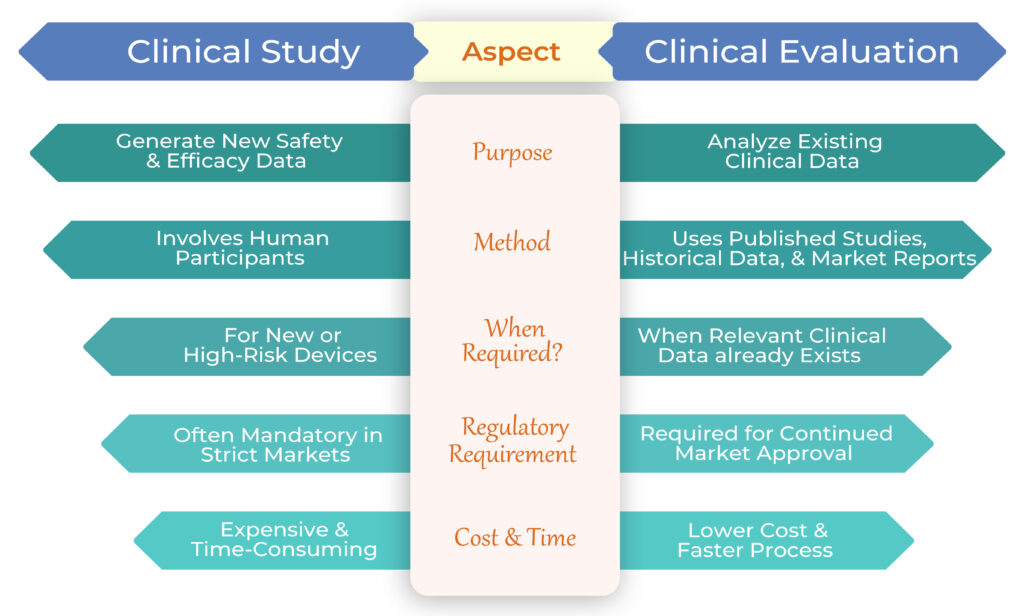
Imagine you’re developing an innovative medical device—one that’s never been used before or has limited existing data on its safety and performance. Regulators will likely require a Clinical Study to gather fresh data on how the device works in real-world conditions.
A Clinical Study involves testing the device on human participants to collect primary data on its safety, efficacy, and performance. This step is often mandatory for cutting-edge medical devices, especially in strict regulatory markets like the EU and the US.
When Do You Need a Clinical Study?
✔ When launching a new or innovative medical device
✔ If there’s insufficient existing data on safety and effectiveness
✔ When entering highly regulated markets like the EU under MDR 2017/745
🛑 Key Point: Clinical Studies take time and require significant investment, but they’re necessary to prove that a device is safe and effective before it reaches patients.
What is a Clinical Evaluation?
Now, let’s say your device is not entirely new, and there’s already relevant clinical data available—perhaps from previous studies, post-market surveillance, or real-world use. Instead of conducting a full-blown Clinical Study, you might only need a Clinical Evaluation.
A Clinical Evaluation is the process of analyzing existing data to demonstrate that your device meets safety and performance standards. It doesn’t involve new human trials but instead gathers information from scientific literature, market data, and historical performance records.
To learn more about clinical evaluation, check out our service page on Clinical Evaluation Reports for Medical Devices!
When is a Clinical Evaluation Sufficient?
✔ If the device has been used before and has supporting clinical data
✔ When there are published studies or reports on similar devices
✔ If post-market surveillance data supports the device’s safety and benefits
💡 Good to Know: A well-structured Clinical Evaluation Report (CER) is mandatory for CE marking under the EU MDR.
Why a Strong Clinical Evaluation Matters for Medical Device Registration
Even if a Clinical Study isn’t needed for your device, a Clinical Evaluation still plays a major role in securing market approval. Regulators want to see proof that the device is safe, effective, and beneficial compared to potential risks.
But here’s the catch—a Clinical Evaluation needs to be thorough, well-documented, and compliant with regulatory expectations. That’s where expert guidance becomes essential.
Need Support With Your Medical Device Registration
How Operon Strategist Can Help
At Operon Strategist, we understand how complex regulatory processes can be, and we’re here to simplify them for you. Our Clinical Evaluation services ensure that your medical device meets all necessary compliance standards—helping you avoid delays and regulatory roadblocks.
✅ We help manufacturers prepare a solid Clinical Evaluation Report (CER)
✅ We analyze existing data, research literature, and post-market insights
✅ We guide you through EU MDR compliance requirements
By now, you should have a clearer understanding of Clinical Studies and Clinical Evaluations. If you’re unsure which one applies to your medical device, reach out to Operon Strategist—we’d be happy to help you find the best regulatory strategy!