Medical devices play a critical role in healthcare, offering lifesaving and life-enhancing solutions to patients worldwide. While many devices are mass-produced to meet general medical needs, some conditions require personalized solutions. This is where custom-made medical devices come into play. These devices are specifically designed and manufactured for individual patients based on their unique anatomical and physiological needs. From prosthetic limbs to customized orthopedic implants, they ensure a precise fit and optimal functionality.
However, due to their individualized nature, custom-made medical devices face unique regulatory requirements, classification challenges, and manufacturing complexities. Navigating these factors is crucial for ensuring both patient safety and compliance with medical regulations.
Looking For a Medical Device Regulatory Consultant?
Let’s have a word about your next project
Key Characteristics of Custom-Made Medical Devices:
- Patient-Specific Design: Crafted based on individual requirements as prescribed by a healthcare provider.
- Not Mass-Produced: Unlike conventional medical devices, these are made in limited quantities or as single-use items.
- Regulatory Flexibility: Subject to different regulatory pathways than standardized devices, allowing more direct manufacturing processes.
Example of a Custom-Made Medical Device:
This product has special design properties, specially adapted and intended to be used only by a given patient to treat their diseases or meet individual needs. An example of a custom-made product is a dental crown, an orthosis, a lower limb prosthesis, or an orthopedic insole.
Regulatory Pathway for Custom-Made Medical Devices
How Are Regulations Different?
Unlike mass-produced medical devices, which must undergo a detailed conformity assessment involving a Notified Body (in the EU) or FDA Premarket Approval (in the USA), custom-made devices follow a different regulatory pathway.
Approval Requirements by Region
- European Union (EU): Custom-made devices do not require Notified Body approval. Instead, manufacturers must maintain a Declaration of Conformity and a comprehensive technical file.
- United States (USA): Custom-made devices are exempt from full FDA approval but must comply with design controls and good manufacturing practices (GMPs).
- India: Custom-made medical devices are regulated under the Medical Device Rules, 2017, where manufacturers must register with the CDSCO (Central Drugs Standard Control Organization) and ensure compliance with safety and quality standards.
- Egypt: The Egyptian Drug Authority (EDA) regulates medical devices, including custom-made ones, under its national framework, ensuring adherence to safety and quality requirements.
- South Africa: The South African Health Products Regulatory Authority (SAHPRA) governs medical devices, including custom-made ones, requiring local licensing and quality assurance compliance.
Challenges in Custom-Made Medical Device Regulation
While custom-made medical devices provide life-changing benefits, they present unique regulatory challenges.
- Documentation Burden
Despite regulatory exemptions, manufacturers must maintain detailed documentation to prove safety, efficacy, and compliance with industry standards.
- Lack of Notified Body Oversight (EU)
Since Notified Bodies do not review custom-made devices, ensuring safety relies heavily on post-market surveillance and manufacturer accountability.
- Timely Accessibility
Custom-made devices need to be produced quickly while maintaining high-quality standards. Balancing speed and compliance are a key challenge in bringing these devices to patients.
Custom-made medical devices offer personalized healthcare solutions but require careful regulatory compliance. While approval processes vary across jurisdictions, manufacturers must ensure safety, efficacy, and timely access to these essential medical innovations. As regulatory landscapes continue evolving, adherence to global standards will be vital for facilitating international market entry and ensuring patient safety.
At Operon Strategist, we play a vital role in supporting manufacturers of custom-made medical devices by providing regulatory consulting services that ensure compliance with the various regional frameworks. Our team of experts assists in navigating complex regulatory landscapes and helps manufacturers meet the stringent safety and quality standards required for custom-made devices.
We specialize in:
- Regulatory Compliance: Helping manufacturers understand and comply with the regulations in different regions such as the EU, USA, India, Egypt, and South Africa.
- Documentation and Technical File Support: Assisting in the preparation of the necessary documentation, including Declaration of Conformity, technical files, and design control records.
- Custom-Made Device Registration: Offering consultancy on the registration processes with regulatory authorities such as the CDSCO in India, the EDA in Egypt, and SAHPRA in South Africa.
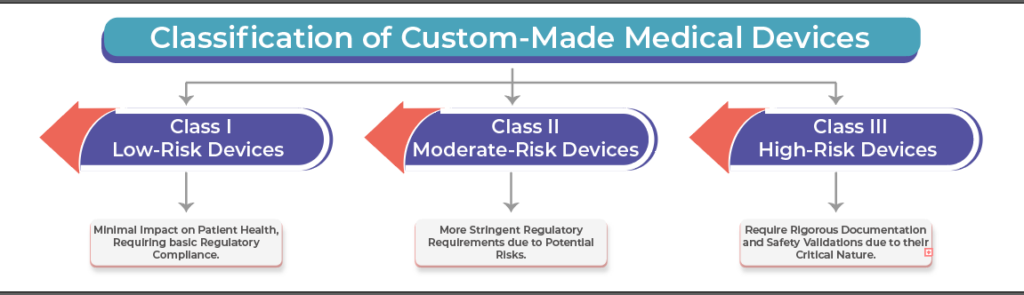
- Risk Management and Safety Validation: Ensuring that custom-made devices meet the required risk classifications and safety validations based on their intended use.
Need expert guidance on custom-made medical device regulations?
Frequently Asked Questions (FAQ)
A custom-made medical device is designed specifically for an individual patient based on a prescription from a healthcare professional. These devices are tailored to address unique medical conditions or anatomical differences.
Yes, custom-made devices must comply with specific regional regulations, though the approval process may differ from that of mass-produced devices. They often require documentation, technical files, and adherence to safety standards.
Generally, custom-made devices can be more expensive because they are manufactured individually or in limited quantities, requiring specialized design, manufacturing, and documentation.
Operon Strategist provides regulatory consulting services for custom-made medical devices, ensuring compliance with regional regulations, assisting in documentation preparation, and offering support in registration and safety validation.




