SFDA Medical Device Registration
SFDA Medical Device Registration Consultants
SFDA (Saudi Food and Drug Authority) medical device registration consultants are specialized professionals who guide manufacturers through the regulatory requirements and approval processes for medical devices in Saudi Arabia.
These consultants possess in-depth knowledge of SFDA regulations, guidelines, and submission procedures. They support manufacturers in understanding compliance obligations, preparing accurate documentation, and meeting the required quality standards necessary for SFDA approval.
By working with SFDA medical device registration consultants, manufacturers can streamline the registration process, reduce regulatory challenges, and improve their chances of obtaining timely approval to market medical devices in the Saudi Arabian market.
SFDA Medical Device Registration An Overview
SFDA is a regulatory authority for medical devices and IVDs sold and distributed in Saudi Arabia. MDMA approval is needed to place your devices in the Saudi Arabia market. SFDA reviews MDMA applications to prepare your submission carefully. Saudi Arabia updated their medical device regulations between 2019 to 2022, which affected device classification and changed the concept and content of MDMA (high-risk application). Due to the changes and updated guidance previously approved devices, IVDs, and medical supplies must comply with new regulations. As medical device regulatory consultants, we always keep an eye on changes in regulations and current updates so that we can serve better to our clients.
Medical Devices Regulations in Saudi Arabia
Before marketing your medical device or in vitro diagnostic device (IVD) in Saudi Arabia, it is essential to register your product with the Saudi Food & Drug Authority (SFDA), the country’s regulatory body for medical devices. Saudi Arabia’s regulations are aligned with the Global Harmonization Task Force (GHTF) guidelines, requiring international manufacturers to obtain permission from at least one GHTF founding member (Australia, Canada, Japan, the European Union, or the United States) before SFDA registration.
Regulatory Authority: Saudi Food & Drug Authority (SFDA)
Regulation: Royal Decree No. (M/54)
Regulatory Pathway: MDMA Approval
Authorized Representative: Saudi Arabia Authorized Representative
QMS Requirement: ISO 13485:2016 certification
50+ Medical Device SFDA Registrations Conquered – Your Gateway to Saudi Arabia Awaits!
Let’s have word about your next project
Medical Device Marketing Authorization (MDMA) registration with the SFDA is required for Medical Devices and In-Vitro Medical Devices (IVD).
Medical Device Marketing Authorization (MDMA)
For marketing devices in Saudi Arabia, all device classifications except for certain categories require Medical Device Marketing Authorization (MDMA) approvals. The SFDA’s registration process for MDMA typically takes an average of 35 days, with licenses valid for either the original term specified or for 3 years if the original duration is indefinite.
What Are the Classifications for Medical Devices in Saudi Arabia?
The SFDA medical device classification is either Class A, B, C, or D. This is according to their risk class. The class is necessary to determine the registration procedure and its requirements.
The SFDA MDS-G5 document details the classification rules (similar to the European MDR classification).
SFDA Medical Device Classification | Risk Class | MDR Classification Rule |
A | Low | I |
A – Sterile | Low-medium | Is |
A – Measuring function | Low-medium | Im |
A – Reusable surgical instruments | Low-medium | Ir |
B | Low-medium | IIa |
C | Medium-high | IIb |
D | High | III |
In the SFDA (MDS-G42) guideline, we can find more clarification of the SFDA classification rules. Concerning in vitro diagnostics, the SFDA is also adopting the European medical device regulation IVDR:
SFDA Medical Device Classification | Risk Class | Classification Rule |
A | Low individual risk and low public health risk | A |
B | Moderate individual risk and/or low public health risk | B |
C | High individual risk and/or moderate public health risk | C |
D | High individual risk and high public health risk | D |
Ready to Revolutionize Healthcare in Saudi Arabia? Register Your Medical Device Today!
Submission of Applications to the SFDA
Your Saudi Authorized Representative is responsible for submitting all application documents to the SFDA for device registration. These submissions are made through the Medical Device Marketing Authorization (MDMA) system in Saudi Arabia.
Documentation Necessary for SFDA Submissions Must Be Provided in English, and Includes the Following:
Details on the manufacturer and the Saudi Authorized Representative
Information about medical devices, such as intended use and labeling/instructions for usage, as well as all marketing materials
Documents proving your market authorization in the GHTF market of the one you prefer.
A statement certifying that the applicant will follow the National Centre of Medical Devices Reporting (NCMDR) requirement that any Field Safety Corrective Action impacting your medical device be notified to KSA authorities.
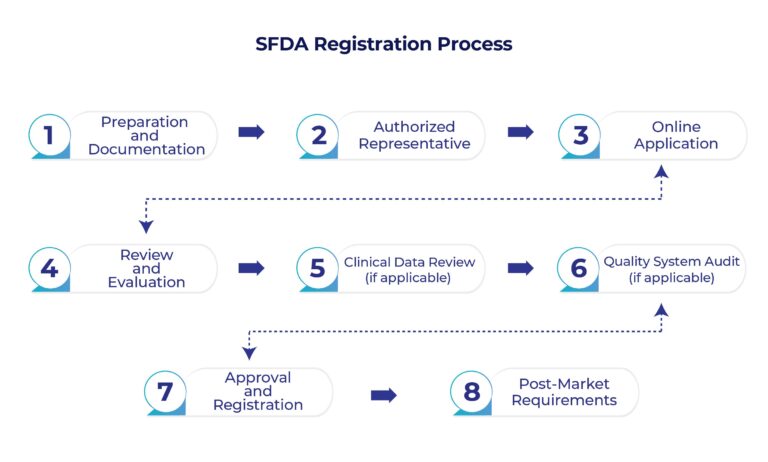
SFDA Medical Device Registration Process
Preparation and Documentation:
– Identify the appropriate medical device classification based on SFDA regulations. The classification will determine the requirements and documentation needed for registration.
– Prepare a complete technical file that includes details about the device’s design, intended use, specifications, labeling, manufacturing process, and more.
– Gather clinical data and evidence of the device’s safety and efficacy, especially if the device is new or has not been previously registered.
Authorized Representative:
– Foreign manufacturers must appoint an authorized representative based in Saudi Arabia. This representative serves as a liaison between the manufacturer and the SFDA.
Online Application:
– Create an account on the SFDA’s electronic submission platform.
– Complete and submit the online application form, providing all required information about the device, manufacturer, and authorized representative.
Review and Evaluation:
– The SFDA will review the submitted documents and information to ensure they meet the regulatory requirements and standards.
– The evaluation may include technical, clinical, and quality assessments of the device.
Clinical Data Review (if applicable):
– If your device requires clinical data or evidence, the SFDA will review this information to assess the device’s safety and effectiveness.
Quality System Audit (if applicable):
– For some medical devices, a quality system audit might be required to assess the manufacturer’s compliance with relevant quality management standards, for example, ISO 13485 Certification
Approval and Registration:
– If the SFDA is satisfied with the documentation, assessments, and audits, they will issue a medical device registration certificate. This certificate allows you to legally market and distribute the medical device in Saudi Arabia.
Post-Market Requirements:
– Once the device is registered, manufacturers must comply with post-market surveillance and reporting requirements, including adverse event reporting.
Obtain Professional Guidance for SFDA Regulatory Compliance Services
How Operon Strategist Can Help You As SFDA Medical Device Registration Consultants?
Operon Strategist supports medical device manufacturers and distributors in navigating the Saudi Food and Drug Authority (SFDA) registration process with ease and compliance.
Our Key Services:
- Regulatory Pathway Guidance: Help with device classification and regulatory strategy.
- MDMA Portal Support: Assistance in registration through the SFDA portal.
- Technical Dossier Preparation: Support in compiling and reviewing SFDA-compliant documents.
- Authorized Representative Services: Help appoint a local representative for foreign manufacturers.
- QMS & ISO 13485 Compliance: Assist in implementing quality systems required by the SFDA.
- Post-Market Surveillance (PMS): Guidance on PMS and vigilance systems.