Manufacturing Plant Facility Layout Design
Medical Device Manufacturing Facility Layout and Project Management Expertise
Consultants specializing in medical device manufacturing facility layout design play an essential role in optimizing the structure and arrangement of facilities. This not only enhances productivity but also ensures safety, compliance, and efficient workflow, which are critical for successful project management in the medical device industry. Engaging experienced consultants, like Operon Strategist, helps create streamlined, compliant, and efficient manufacturing environments essential for the high standards required in medical device project management.
Designing Manufacturing Facilities for Medical Devices in Saudi Arabia
As Saudi Arabia’s healthcare sector rapidly advances, the Kingdom has become a significant market for international medical device manufacturing. Establishing a facility in Saudi Arabia requires a detailed and compliant plant layout, particularly due to regulatory standards from authorities such as the US FDA, CDSCO, SFDA, and others. For medical device contract manufacturing companies, compliance with these regulations is non-negotiable.
Manufacturers of diverse medical devices, including orthopedic implants, disposables, and primary packing materials, must design their manufacturing plants following cGMP and other regulatory standards. A well-structured layout enables efficient movement of personnel and materials, minimizes contamination risks, and meets all regulatory and medical device contract manufacturing requirements.
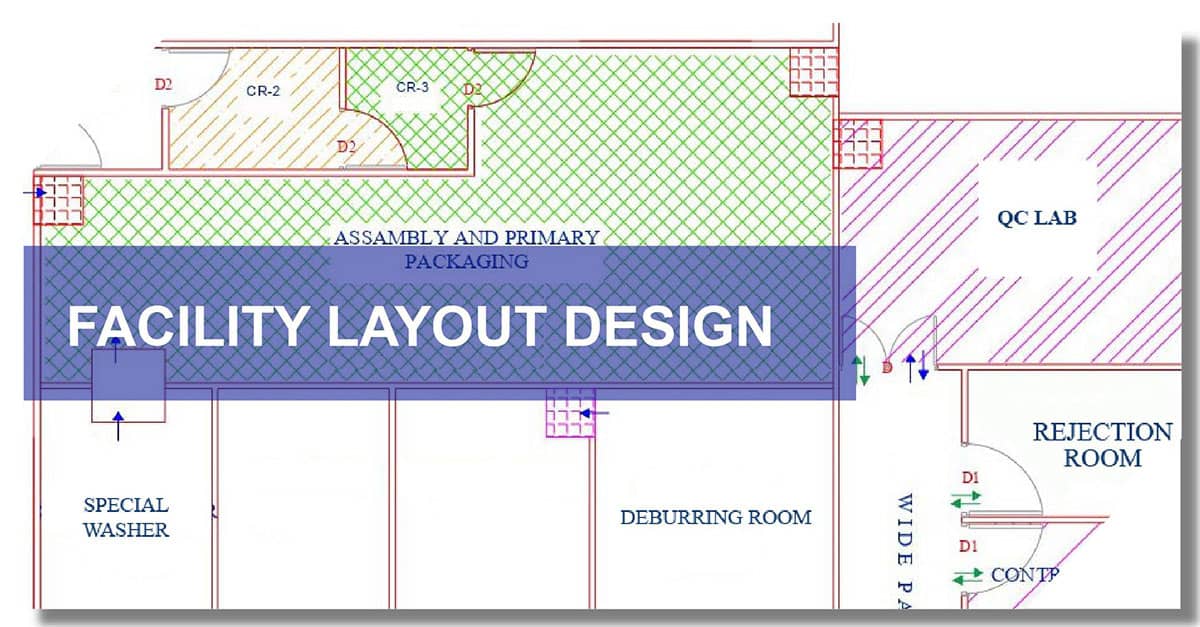
The effective design of a facility is crucial, taking into account the smooth movement of both people and materials. It involves several considerations such as product segregation, manufacturing workflows, procedural steps, and the utilization of designated areas. Expert guidance is invaluable in making informed decisions, facilitating a seamless transition during regulatory assessments, and meeting customer inspection standards.
Our expertise lies in devising manufacturing plant layouts using our skilled Auto-CAD professionals, who collaborate closely with clients and their architects or civil engineers. This meticulous planning phase aids in determining the spatial needs for various processes and accompanying equipment. Our designs are strategically crafted to ensure a unidirectional flow of individuals and materials, minimizing the risk of cross-contamination. Moreover, we offer insightful recommendations to manufacturers regarding future expansions or alterations, tailoring the layout to optimize space usage and enhance overall efficiency.
Looking for Medical Device Regulatory Consultants?
From Start to End Services as Project Management in the Medical Device Industry
How We Help Manufacturers in Manufacturing Plant Layout Design:
Achieving compliance is best demonstrated through the right design. A well-crafted design minimizes manual errors and reduces the effort needed to control activities. Therefore, the proper design of the product, manufacturing site, and the system is pivotal in ensuring product quality and preemptively addressing potential issues stemming from poor designs.
Operon Strategist diligently studies the client’s requirements, product specifics, and the manufacturing flow process to create an appropriate facility layout design. This design is meticulously tailored to meet regulatory requirements such as those from SFDA, ensuring the unidirectional flow of personnel and materials, preventing cross-contamination, and correctly assigning clean room classifications, among other crucial considerations.
Know regulatory requirements for SFDA registration

There are various expectations “implied “and “expected to be understood” laid down by the regulators across the world. Some of the expectations can be well understood and compiled in the facility based on the experience of the regulatory audits. In the case of the existing facility, it is very important to know the facility compliance before applying for the regulatory audits to avoid irreversible losses at the end of the facility audit.
As experienced medical device regulatory consultants, our manufacturing plant layout design is appreciated by various regulators for their compliance and for minimal man and material movements and product segregation.
