Medical Device Product Feasibility & Detail Project Report
Creating a comprehensive Medical Device Product Feasibility & Detail Project Report requires a diverse range of skills spanning engineers, regulatory experts, market analysts, project managers, and financial professionals. Serving as a comprehensive roadmap, this report provides invaluable insights for stakeholders, investors, and development teams, ensuring a systematic approach from concept to market launch. Operon Strategist is poised to provide expert support throughout this multifaceted process, facilitating a smooth journey toward successful medical device development and market entry.
Market Analysis and Product Feasibility for Medical Devices
Market analysis and feasibility for the Medical Devices product development cycle must be the first critical stage of any project. For the concept to be effectively communicated, it is important to understand what the client wants and characterize the product.
To create technology for product development and commercialization, a regulatory pathway must be resolved. A Quality Management System (QMS) including risk management and the executives arranging is vital. The product design idea must be assessed to decide clinical utility and gauge costs. Clinical significance is resolved to safeguard advertise acknowledgment. The identification of necessities has been demonstrated to save time and money in product development by controlling the development effort.

Operon Strategist is a medical device regulatory consulting company that provides regulatory advisory & guidance to various manufacturers in the healthcare industry to ensure the strategic development of these manufacturers.
About Market Research, Market Analysis and Product Feasibility for Medical Devices
Conducting thorough market research is essential. Prices for the same device can vary across different countries and regions, but it goes beyond mere number-crunching. Cultural considerations play a significant role. Some cultures may have biases towards or against specific medical procedures or devices.
Understanding the size of your target market holds crucial importance for various reasons. If a market is too small, it’s vital to recognize early on that pursuing it may not be worthwhile. Moreover, market size influences the amount of capital you can potentially raise if you opt for that route. While assessing the size of certain individual markets can be based on existing data, gauging international markets presents greater challenges.
Seeking Market Analysis and Product Feasibility for Your Medical Device?
Two Major Segments for Medical Device Manufacturing.
a) Electrical devices: like ECG machines, Pulse oximeter machines, and X-rays.
b) Disposable devices: like syringes, Masks, IV cannulas, IV sets, etc.
For marketing and feasibility, depending on the choice, we might require using different pools of expertise. This can also be decided on the area of expertise, like if electronics then with the electronic regime or if any molding, plastic expertise in disposable products.
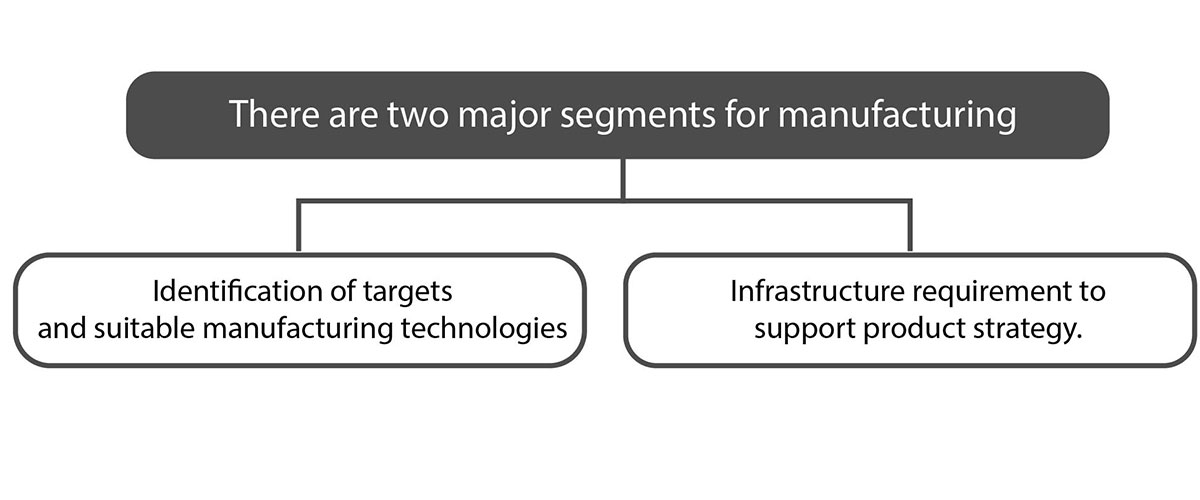
1) Identification of targets and suitable manufacturing technologies
Medical device manufacturers aim to enhance efficiency and speed in their manufacturing processes, all while upholding corporate responsibility. This necessitates a continuous focus on renewable resources, sustainable materials, energy-efficient equipment, appropriate manufacturing technologies, and waste reduction methods.
2) Infrastructure requirement to support product strategy
The initial stages of development involve essential tasks like establishing device specifications, identifying intended uses and indications, and compiling pertinent data to ensure product safety for market and feasibility assessments. A comprehensive risk analysis is conducted to bolster the product’s global development strategy. The infrastructure is contingent upon manufacturing location and target market, especially for products that are serializable or disposable.
How Can Operon Strategist Guide You in Market Analysis?
We serve our clients by providing turnkey services, system implementation, training, licensing, regulatory approvals, and certifications. Operon will provide you complete guidance on with the market analysis and feasibility of the medical devices, and the complete product development process.
Contact us for more details. We also provide medical device regulatory consultation for India, South Africa, Egypt, the USA, the UK, Costa Rica, Oman, and Iran.
FAQs
What is a feasibility study medical device?
An early feasibility study (EFS) is a small clinical assessment of a device that is still in the early stages of development. It usually enrols a limited number of individuals, is intended to assess the device design idea in terms of initial clinical safety and device functionality, and may lead device revisions.
What is product feasibility report?
A New Product Feasibility Study evaluates a new product idea's marketability. It is carried out prior to the expenditure of major resources on product development and marketing. The study serves as a road map, indicating whether the new product should be developed and launched.
What is the feasibility report of a manufacturing company?
A manufacturing plant feasibility report includes an evaluation and assessment of a proposed project to see whether it is feasible to manufacture the product to meet customer needs.
