Medical Device Process Validation Consultant
A Medical Device Process Validation Consultant specializes in ensuring the efficacy, reliability, and compliance of manufacturing processes used in the production of medical devices. Process validation is a critical aspect of the medical device industry to confirm that manufacturing processes consistently produce devices meeting predetermined quality standards. Operon Strategist can provide quality assistance for the same.
What is Medical Device Validation?
Medical Device Process Validation is a process of establishing documentary evidence demonstrating that a procedure, process or activity carried out in production maintains the desired level of compliance at all stages. In straightforward words, the interaction approval for a medical device is the assortment and assessment of information, from the cycle configuration stage till the creation, as it gives logical proof that the cycle is able to do reliably giving quality items. In simple words, the process validation medical device is the collection and evaluation of data, from the process design stage till the production, as it gives scientific evidence that the process is capable of consistently providing quality products.
Why Are Medical Device Utilities and Equipment Validation Required?
The medical device validation process ensures that medical devices that are manufactured will perform safely and appropriately. The regulatory bodies are setting a few standards for the validation and verification of the devices. As medical device regulatory consultants, we provide guidance to create validation reports as per regulatory requirements.
What Is the Difference Between Process Verification and Validation?
When a manufacturer plans to introduce a medical device in the market, they should have a high degree of certainty that their process has proper control while producing the products and they meet the specified regulatory criteria. The USFDA and ISO 13485 require manufacturers to verify their product meets the design specifications and this can be accomplished by testing or inspecting it. This is a verification process that means verifying the quality of the product but testing every single device is impractical, and process validation comes into the picture. The process validation completes the quality assurance need. As an ISO 13485 medical device consultant We know the guidelines provided by the standard and we help manufacturers to implement them correctly.
According to USFDA process validation means establishing the objective evidence that a process consistently meets the product’s Predetermined specifications.
Why Do Medical Device Manufacturers Need a Validation Process?
Medical device manufacturing process validation is a necessary part of regulatory agencies like the US FDA which has long introduced the validation process as a significant requirement of quality assurance for medical device manufacturers.
- The medical device validation process ensures that you have objective evidence that meets the user’s needs and their intended use. It is performed by tests and inspections.
- Generally, the motive of validation is to make sure that the user’s needs are met in medical devices that consistently provide the intended medical benefits in the actual–use medical conditions.
- Process validation, as the name implies, focuses on the production of the device. The reason for this is to satisfy the FDA’s requirements and ensure business success.
- Prior to presenting another medical device onto the market, makers ought to have a serious level of sureness that their assembling processes have the appropriate controls set up to create items that are protected and meet indicated client, specialized, and administrative necessities.
How Is the Process Validation Done by Medical Device Consultants?
Process validation is defined as the collection and evaluation of information from the process of the design stage throughout the production, which gives scientific proof that this process is capable of consistently delivering quality products. Medical Device Design validation focuses on the device itself and involves creating evidence that it meets the user’s needs and their intended uses. It is done on the first batch of production units after the prototype, that is before the mass production of the unit begins.
A team of Quality assurance, engineering, and manufacturing is required for process validation. It is done at the user’s place and not controlled but the lab conditions. The entire unit of packaging and labeling is involved. Both regulations, 21 CFR Part 210 and 211, specify that the Equipment and Utilities Validation requires qualification when they have direct product impact. Qualification testing provides you with the assurance that your equipment is operating properly and meets both the user and product – requirement specifications.
Medical Device Process Validation Services:
Medical Device Process Validation is an important cGMP requirement & many regulatory agencies audit the validation documentation. Validation of a machine or a process is carried out to ensure that products of consistent quality are manufactured and the required level of compliance is met at every stage. Since a wide variety of procedures, processes & activities need to be validated.
Looking for Medical Device Regulatory Consultants?
Medical Device Process Validation Is Divided Into the Following Sub-Sections:
- HVAC validation
- Equipment validation
- Process validation
- Facilities validation
- Cleaning validation
- Analytical method validation
- Personnel validation
- Packaging validation
- Computer system validation
During medical device process validation studies, it is very important to have the predefined protocol stating the details of the activities performed and pre-defined acceptance criteria. After the conclusion of the qualification, the statement shall be made to guide the routine processing parameters which have to be a monitoring parameter during the routine manufacturing run. The output of the validation shall be made the input for the routine control of the machines and utilities. The initial qualification of the machine/utilities may be exhaustive for machine acceptance and parameter settings. The routine qualification may be little less exhaustive than that of the initial qualification and it has to be reported in the formal report.
How Will an Operon Strategist Help in Process Validation in Medical Device Manufacturing?
Being a medical device consultant We provide medical device process validation to manufacturers & medical device process validation service providers for the validation activity & documentation in Saudi Arabia. We can also provide guidance on regulatory requirements for the process. The product and the production facility are studied to document the validation master plan according to which validation activities will be performed as per the defined timeline. We have an experienced team of people who understand the technical functionality of the process. Our team works closely with each client to tailor validation projects as per their exact needs.
Why Choose Operon Strategist as a Validation Process Consultant?
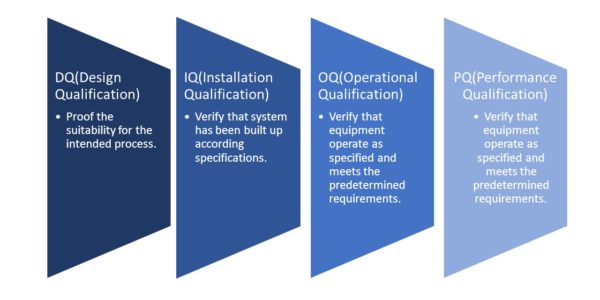
We provide guidance for preparing FDA medical device process validation protocols & reports in the correct format so as to meet the regulatory requirements including IQ (installation qualification), OQ (operational qualification), PQ (performance qualification) DQ (design qualification) protocols & PQ report, we also provide guidance on EU MDR medical device process validation.
We have validation experts who guide you in design qualification and site acceptance and also provide validation consulting for facility and utility qualification, medical device manufacturing and packaging validation, design validation, etc. For more details on validation services please contact us. Our global presence adds the experience of working with various regulatory bodies, If we particularly talk about the Saudi Arabia region our satisfied customer base inspires us to provide more and more quality services.
We also provide medical device consultation for India, South Africa, Egypt, the USA, the UK, Costa Rica, Oman, and Iran.
