As the medical device industry faces stricter global regulations in 2025, the importance of the process of validation in medical devices continues to grow. Medical device manufacturers must now prove — with clear, documented evidence — that their processes consistently produce safe, effective, and compliant products.
With authorities like the FDA, EU MDR, and local regulators such as the Egyptian Drug Authority (EDA) increasing scrutiny, ensuring effective process validation isn’t optional. In this blog, we explain the evolving expectations for medical device process validation, 2025 compliance trends, and how manufacturers can safeguard product quality and patient safety.
Looking For a Medical Device Regulatory Consultant?
What Is Process Validation in Medical Devices?
manufacturing process, when operated within established parameters, can reliably produce products meeting predefined specifications and quality attributes.
It’s especially vital for processes where end-product testing alone cannot confirm safety and efficacy — such as sterilization, software-controlled manufacturing, and cleanroom operations.
Why Validation in Medical Devices Matters in 2025
In 2025, regulations like FDA’s Quality System Regulation (QSR), EU MDR, and anticipated updates in ISO 13485:2025 demand continuous, data-driven validation strategies. Manufacturers must demonstrate not only initial process validation but also consistent ongoing monitoring and risk-based revalidation.
Key drivers include:
- Growth in connected, software-driven, and AI-enabled medical devices
- Enhanced global regulatory oversight and audit frequency
- Expanded requirements for software and automated system validation
- Increased focus on continuous process monitoring and data integrity
Regulatory Expectations for Process Validation in 2025
The 2025 regulatory environment focuses on:
- Lifecycle validation covering design, initial validation, continuous monitoring, and revalidation
- Data-driven Continuous Process Verification (CPV) using real-time manufacturing data
- Integrated software validation for systems affecting product quality and safety
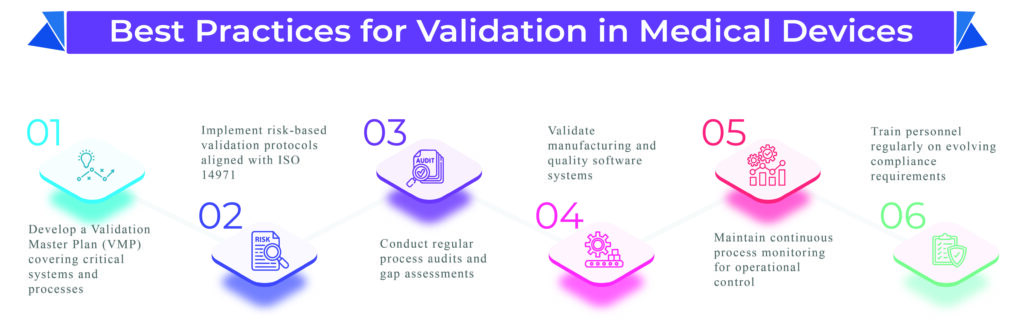
- Risk-based validation planning aligned with ISO 14971
- Digital traceability and documentation management ensuring audit readiness
Key Stages of Validation in Medical Devices
The validation lifecycle typically includes:
- Process Design
- Define critical process parameters and conduct risk analysis
- Define critical process parameters and conduct risk analysis
- Process Qualification (IQ, OQ, PQ)
- Installation Qualification (IQ)
- Operational Qualification (OQ)
- Performance Qualification (PQ)
- Installation Qualification (IQ)
- Continuous Process Verification (CPV)
- Real-time monitoring, statistical process control (SPC), and deviation management
- Real-time monitoring, statistical process control (SPC), and deviation management
- Revalidation
Required after changes in equipment, processes, or regulatory updates
Common Validation Challenges and Solutions
Frequent issues manufacturers face:
- Incomplete documentation or outdated protocols
- Lack of software validation for digital systems
- Weak revalidation strategies
- Insufficient real-time process data for CPV
Solution: Adopt a comprehensive, lifecycle-based validation model supported by clear documentation, risk assessment, and digital data analysis.
Request Expert Support for Process Validation, Regulatory Compliance, and Licensing in Medical Device Manufacturing
How Operon Strategist Supports Validation in Medical Devices?
At Operon Strategist, we offer specialized, end-to-end consulting for validation in medical devices, designed to help manufacturers meet global and regional regulatory demands. Our experts develop Validation Master Plans (VMP), conduct IQ, OQ, PQ protocols, and set up Continuous Process Verification (CPV) systems for consistent product quality.
We also assist with:
- Software validation for production and quality systems
- Cleanroom, sterilization, and packaging process validations
- Risk assessment and validation gap analysis
- Revalidation planning for process or regulatory changes
Our services align with FDA QSR, EU MDR, ISO 13485:2025, MDSAP, and EDA Egypt guidelines, ensuring your operations remain audit-ready and compliant.