FDA 21 CFR Part 820 Quality System Regulation
FDA 21 CFR Part 820 Compliance Consulting Services
FDA 21 CFR Part 820, also known as the Quality System Regulation (QSR) for medical devices, outlines the requirements for manufacturers to establish and maintain a quality management system (QMS). This ensures the safety, efficacy, and regulatory compliance of medical devices sold in the U.S. market.
An Overview of FDA 21 CFR Part 820
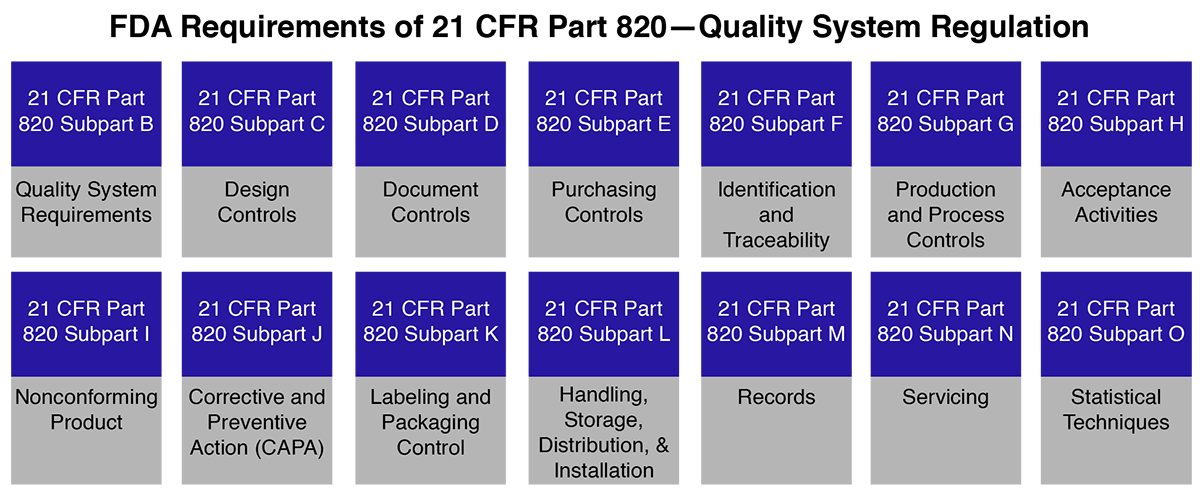
FDA 21 CFR Part 820 establishes current good manufacturing practice (CGMP) requirements for medical devices. It covers critical areas such as design, manufacturing, packaging, labeling, storage, installation, and servicing. Manufacturers must align their quality systems with these regulations to ensure compliance and seamless FDA inspections.
What is FDA 21 CFR Part 820 Quality System Regulation?
First, let’s clarify CFR, which stands for Code of Federal Regulations. In CFR, 21 represents the title, where 800 pertains to medical devices, and 820 specifically addresses Quality System Regulation (QSR).
FDA 21 CFR Part 820 governs the processes, facilities, and controls involved in designing, manufacturing, packaging, labeling, storing, installing, and servicing medical devices. Manufacturers undergo inspections by the US FDA based on Part 820 requirements, with compliance being assessed rather than certified.
FDA QSR Compliance for Medical Device Manufacturers
21 CFR Part 820 is integral to the Current Good Manufacturing Practices (CGMP) regulations, providing a robust regulatory framework. CGMP governs quality systems across a spectrum of FDA-regulated products, including food, drugs, biologics, and medical devices. Specifically for medical devices, the meticulous standards are encapsulated in 21 CFR Part 820, commonly known as the Quality System Regulation (QSR) by the FDA.
These stringent requirements, mandated under section 520(f) of the Federal Food, Drug, and Cosmetic Act, were initially established to ensure the quality and safety of medical devices manufactured under FDA oversight in the United States. 21 CFR Part 820, or QSR, outlines the current good manufacturing practices essential for the production of medical devices in the U.S.
Looking For Regulatory Consultants in Saudi Arabia?
Let’s have a word about your next project!
The FDA’s Quality System Regulation (QSR), detailed in 21 CFR Part 820, comprehensively delineates directives and regulations that govern various aspects of medical device production. This encompasses the entire process from manufacturing through labeling, packaging, storage, installation, design, and servicing of finished medical devices intended for human use and distribution within the United States.
These stringent standards are crucial for ensuring the safety and effective production of medical devices. Manufacturers in the medical device industry must strictly adhere to these guidelines to facilitate FDA inspections and ensure compliance with FDA QSR 21 CFR Part 820. Ultimately, this adherence guarantees the quality and safety of medical devices available in the American market.
Why Should You Care About 21 CFR Part 820?
Adhering to the regulations outlined in 21 CFR Part 820 is paramount for medical device manufacturers. Non-compliance with these essential standards during an FDA inspection may lead to the issuance of a warning letter, carrying the potential for significant reputational damage and adverse effects on market performance.
For those involved in importing medical devices from the United States to Saudi Arabia, a thorough assessment of product adherence to Quality System Regulation (QSR) requirements is strongly recommended. Ensuring compliance is not only a regulatory obligation but also a vital measure to guarantee the safety and quality of the devices introduced to the Saudi Arabian market.
Do You Need Consultation for FDA CFR Part 820?
Our Role in FDA 21 CFR Part 820 – Quality System Regulations:
For companies aiming to enter the U.S. market with medical devices, strict compliance with the US FDA Quality System Regulation (QSR), specifically 21 CFR Part 820, is an absolute necessity. Even if your organization already has a quality management system in place, it must be harmonized with this regulation before your devices can be introduced into the U.S. market.
If you are a medical device manufacturer in Saudi Arabia with ambitions of expanding into the U.S. market, adhering to U.S. regulatory requirements is a critical and foundational step. Operon Strategist, our dedicated team, is well-prepared to assist you throughout the entire process of achieving compliance.
Our approach begins with a thorough gap analysis of your existing system, providing a precise assessment of the current state of your quality system. We offer specialized 21 CFR Part 820 training courses that guide clients in creating and implementing the necessary documentation to meet the regulation’s requirements across various functions within your organization. Additionally, we conduct mock audits to evaluate the effectiveness of your 21 CFR Part 820 implementation.
Our commitment extends to offering post-inspection guidance and helping you address any identified non-conformances during the audit process. By aligning with FDA 21 CFR Part 820, manufacturers can establish and follow robust quality systems, ensuring that their products consistently meet the required standards and specifications. This, in turn, paves the way for a successful entry into the U.S. medical device market.
Why Choose Operon Strategist?
Operon Strategist is an FDA 510 k Clearance Consultant and helps clients to register SBU (Small Business Units), if applicable. Take out the testing requirement of the product, creation of the dossier, resolving the queries and after completion of all the activities, the client receives the US FDA 510 k approval.
We are a medical device consulting firm assisting companies and medical device manufacturers by providing consultancy services that support the registration of drug-device combination products. We have experience with each constituent part and the GMP regulations that together form the basis for their development and manufacture: Drug (21 CFR 210 and 211), Device (FDA 21 CFR part 820), and Combination Products (21 CFR Part 4). Our experience and work methodology differentiated us from others. To know more details and to avail yourself of our services you easily contact us.
We also provide medical device consultation for India, South Africa, Egypt, the USA, the UK, Costa Rica, Oman, and Iran.
FAQs
What is the difference between ISO 13485 and 21 CFR Part 820?
ISO 13485 is an internationally accepted standard that offers an approach to abide by common regulatory requirements. Other countries have their own regulations; FDA 21 CFR Part 820 is solely a U.S. regulation. ISO 13485 standards have undergone multiple revisions.
What is Design Control for medical device?
Design Control labels the implementation of a formalistic technique to the ways of product development activities. It is often necessary by regulation for the appliance of such practice when designing and developing products within regulated industries e.g. medical devices. The Food and Drug Administration FDA has stated that medical device manufacturers that want to marketized certain classifications of medical devices in the USA follow design control requirements (21 CFR 820.30). Few firms regard Risk Management and Design Control as they are linked together but they follow separate procedures, not actualizing that the relation between the user needs, design inputs, dangers and dangerous circumstances. Design control conditions that when the suppliers or manufacturers get a product to design controls they may discover and maintain the correct documentation to assure the specified design requirements are.
What is 820.30 FDA regulation?
820.30 Design controls. (a) General. (1) Each manufacturer of any class III or class II device, and the class I devices listed in paragraph (a)(2) of this section, shall establish and maintain procedures to control the design of the device in order to ensure that specified design requirements are met.