Clean Room Design Consultant for Medical Devices
Clean rooms are critical for maintaining sterility, reducing contamination, and ensuring product safety in medical device manufacturing. As a trusted Clean Room Design Consultant for Medical Devices, Operon Strategist provides comprehensive design, validation, and regulatory solutions tailored to international standards. Our expertise ensures your facility operates efficiently, complies with ISO 14644-1, and delivers high-quality, safe medical devices.
Why Medical Device Manufacturers Need Clean Rooms
Clean rooms create a controlled environment free from dust, microbes, and aerosol particles, which is critical for producing reliable and safe medical devices. Key benefits include:
- Minimized Contamination: Maintain an environment with low pollutant levels to prevent product defects.
- Regulatory Compliance: Meet stringent standards such as ISO 14644-1 and other global regulations.
- Enhanced Product Safety: Guarantee sterility, efficacy, and quality of medical devices.
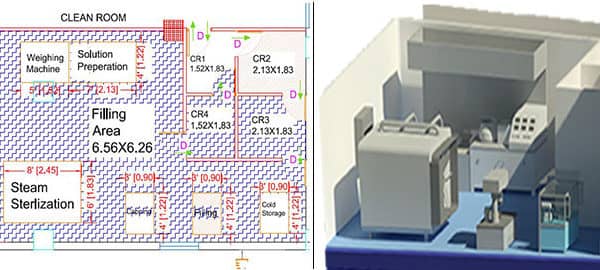
Clean Room Design Requirements for Medical Devices
To ensure optimal performance, clean rooms must meet specific requirements:
Air Quality: Use HEPA filters to remove particles as small as 0.3 microns; maintain positive air pressure to prevent contamination.
Temperature & Humidity Control: Maintain 18–26°C and relative humidity between 30–70%.
Gowning Procedures: Mandate cleanroom garments and controlled entry/exit to reduce contamination from personnel.
Layout Design: Include designated zones for gowning, storage, sterilization, and waste disposal to prevent cross-contamination.
Surface Cleanliness: Use smooth, crack-free surfaces for easy cleaning and disinfection.
Sterilization Areas: Dedicated zones for sterilizing medical devices to maintain product safety.
Monitoring Systems: Regularly test air quality, temperature, and microbial levels to ensure ongoing compliance.
Regulatory Guidance for Clean Rooms
Operon Strategist ensures your clean room design meets regulatory standards across countries such as Costa Rica, South Africa, Egypt, Oman, UK, USA, Saudi Arabia, and Iran. We provide guidance on:
- Entry and exit protocols
- Gowning and de-gowning procedures
- Air circulation systems to maintain ISO compliance
- Regulatory approvals and documentation support
Operon Strategist: Your Clean Room Design Partner
- Custom Clean Room Design: Tailored layouts and HVAC systems for efficient, sterile production.
- Validation & Compliance: ISO 14644-1 support, from installation to operational qualification.
- Regulatory Guidance: Assistance with local and international approvals (FDA, EU MDR).
- Industry Expertise: Solutions for manufacturing, R&D labs, and packaging industries.
- Advanced Tools: AutoCAD designs for precision, airflow optimization, and contamination control.
Ready to Build Your ISO-Compliant Clean Room?
Our Role in Clean Room Conceptualization & Regulatory Approvals
- We as Clean Room Design Consultants, guide medical device manufacturers on supporting elements to maintain their clean room conditions suitable for manufacturing like entry-exit procedures, gowning procedures etc.
- As the clean room design consultants, we need to ensure the various sizes and unpredictability which are required for structuring and to keep up low degrees of air particles according to the clean room standard ISO 14644-1.
- Clean room design is important to consider the whole air circulation framework, including arrangements for sufficient, downstream air returns. In vertical stream rooms, this implies the utilization of low-divider air returns around the edge of the zone.
Operon Strategist, a medical device regulatory consulting company also provides regulatory advisory & guidance to various manufacturers in the healthcare industry to ensure the strategic development of these manufacturers. We also serve clients for Cleanroom projects in Costa Rica, the UK, Oman, South Africa, Saudi Arabia, the USA, Iran, and Egypt. Feel free to contact us for more info.

FAQs
What are ISO standards for clean rooms?
The ISO 1 specification for cleanrooms requires less than 2 particles greater than 0.3 microns and no particles greater than 1.0 microns per cubic meter. The ISO 2 specification for cleanrooms requires less than 11 particles greater than 0.3 microns and no particles greater than 1.0 microns per cubic meter.
How are clean rooms classified as per ISO Standards?
Medical device manufacturing typically performs in an ISO 5 (Class 100) to ISO 8 (Class 100,000) cleanroom. Medical device packaging typically is conducted in an ISO 7 (Class 10,000) or ISO 8 (Class 100,000) cleanroom with an ISO 8 (Class 100,000) gowning room.