Why 3-Way Stopcock Manufacturing Matters
3-way stopcock manufacturing plays a vital role in the medical device industry, ensuring patient safety, effective fluid management, and regulatory compliance. These small yet essential devices are widely used in hospitals, clinics, and emergency care units to control the direction of fluids in IV lines.
If you are a manufacturer aiming to enhance production efficiency or a healthcare professional interested in understanding stopcock variants, this comprehensive guide will help you navigate every aspect—from materials and designs to certifications and regulatory approvals.
Boost your 3-way stopcock manufacturing project with Operon Strategist’s expert solutions tailored to your business needs.
Looking for a Consultation on Medical Device Registration?
What is a 3-Way Stopcock?
A 3-way stopcock is a medical valve that regulates fluid flow in IV systems. It has three ports and can be used to:
- Close the flow completely (off position)
- Connect two lines for continuous infusion
- Add medication or fluids through a third port
These devices are indispensable in managing fluids and ensuring efficient treatment delivery during medical procedures. High precision and consistent manufacturing are crucial to maintain patient safety and meet industry standards.
Types of Medical Stopcocks and Their Applications
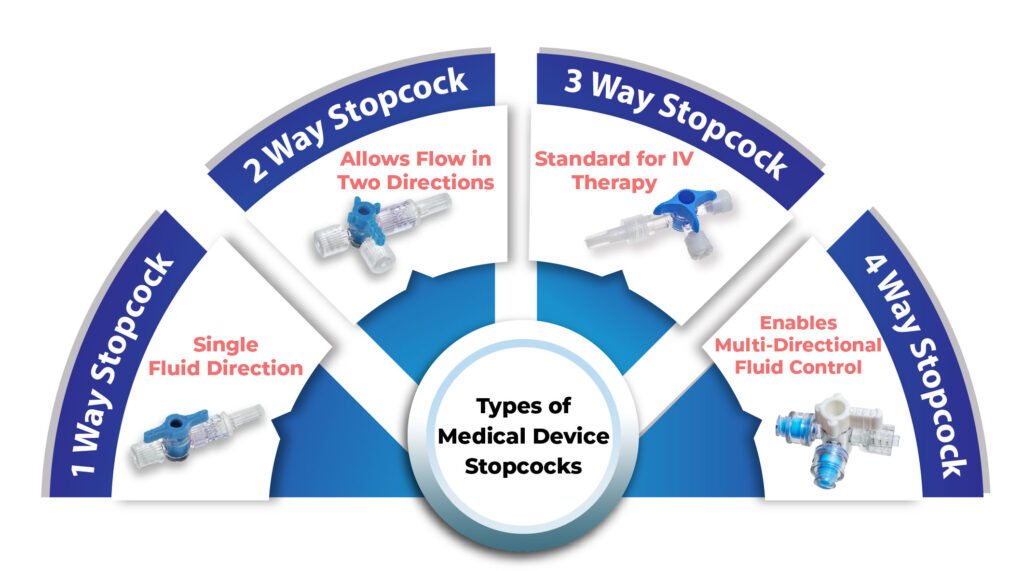
Stopcocks are available in various configurations depending on medical requirements:
1-Way Stopcock – Allows fluid flow in a single direction
2-Way Stopcock – Controls flow between two pathways
3-Way Stopcock – The most common model used in IV therapy
4-Way Stopcock – Offers multi-directional flow control for advanced setups
For complex medical procedures, manifold stopcocks with 2 to 6 gang configurations provide greater flexibility in connecting multiple lines.
Connection Types
- Female luer lock
- Male luer lock
- Tubing ports
- Male luer slip
Choosing the right connection depends on the intended medical procedure, pressure handling, and compatibility requirements.
Boost your 3-way stopcock manufacturing with expert plant layout and turnkey solutions. Contact Operon Strategist today!
Material and Design Specifications for 3-Way Stopcock Manufacturing
High-quality 3-way stopcocks must meet stringent material and design criteria to ensure safety and reliability:
- Biocompatible Polycarbonate or Polysulfone – Offers strength and transparency for fluid monitoring
- Single-Use, Sterile, Non-Pyrogenic – Ensures infection-free usage
- Pressure Resistance Up to 4.5 Bar – Handles varying flow rates and pressure needs
Available Variants
✔ Lipid-resistant, BPA-free formulations
✔ Color-coded handles (Red, Blue, White) for easier identification
✔ Tyvek packaging for maintaining sterility
Color-Coding Uses
- Blue Tap – Venous procedures
- Red Tap – Arterial use
- White Tap – Special cases
These thoughtful design elements enhance safety, simplify operation, and ensure compatibility across medical settings.
Regulatory Compliance: Meeting Global Standards
Manufacturers must strictly adhere to guidelines to ensure product safety and market access. The key regulatory frameworks include:
- CDSCO Registration (India) – Mandatory for local market approval
- ISO 13485 Certification – Ensures global quality management compliance
- EUDAMED Registration – Required for EU Medical Device Regulation adherence
Documentation Requirements
- Technical file preparation
- Clinical evaluation reports
- Manufacturing process validation
Foreign manufacturers must appoint an Indian Authorized Representative to facilitate compliance and distribution within India.
The 3-Way Stopcock Manufacturing Process: Step-by-Step
Here’s a typical process involved in manufacturing high-quality 3-way stopcocks:
1. Material Procurement
Select medical-grade polycarbonate or polysulfone based on application requirements and biocompatibility standards.
2. Molding and Injection
The stopcock body is formed using precision injection molding machines, ensuring uniformity and defect-free components.
3. Cleaning and Surface Treatment
Post-production cleaning eliminates impurities and prepares surfaces for sterilization and labeling.
4. Assembly and Testing
Ports, seals, and handles are assembled in cleanroom conditions. Rigorous testing ensures that the stopcock withstands pressure and maintains integrity during medical use.
5. Sterilization and Packaging
Stopcocks are sterilized using validated methods and packaged in sterile containers to ensure safe transportation and storage.
6. Quality Control and Final Inspection
Each batch undergoes strict quality assurance checks, including leak tests, pressure validation, and visual inspections before market release.
Ready to optimize your 3-way stopcock manufacturing process?
How Operon Strategist Can Help
At Operon Strategist, we support manufacturers at every stage of the 3-way stopcock manufacturing process:
✔ Turnkey Project Solutions – From plant layout to commissioning
✔ Regulatory Consultation – CDSCO registration, ISO 13485, CE marking, and more
✔ Technical Documentation – Assisting in file preparation, validation, and approvals
✔ Process Optimization – Enhancing manufacturing efficiency and reducing waste
With over a decade of expertise, we provide comprehensive solutions that ensure your products meet global standards.




