e-QMS Implementation: An Overview
Implementing an electronic Quality Management System (e-QMS) is a transformative step for medical device manufacturers seeking to enhance operational efficiency, ensure regulatory compliance, and maintain superior product quality. Whether transitioning from a manual paper-based system or upgrading an outdated e-QMS, a well-planned approach is crucial to avoid disruptions and maximize benefits. By carefully structuring the implementation process, companies can streamline document control, training management, risk assessment, and compliance tracking, leading to improved productivity and a stronger quality culture.
Looking For a Medical Device Regulatory Consultant?
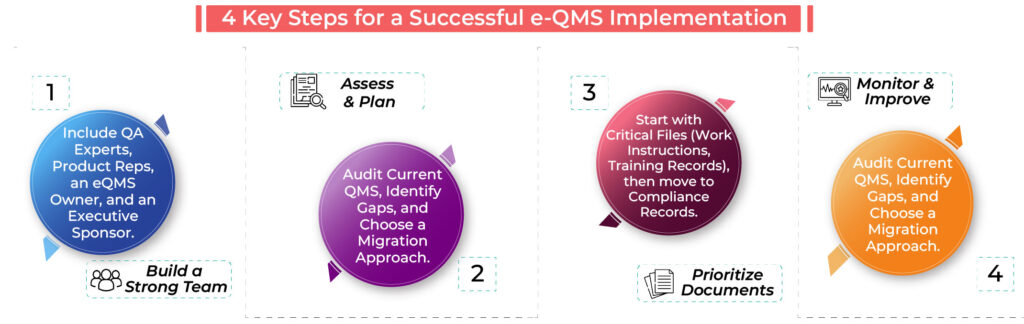
1. Build a Strong e-QMS Implementation Team
Rolling out an e-QMS isn’t a one-person job—it requires a dedicated team to ensure a seamless transition. Instead of putting all the responsibility on one person, bring together a cross-functional team that includes:
✅ Quality assurance experts – to ensure compliance with regulations.
✅ Product development representatives – to align processes and workflows.
✅ A dedicated e-QMS owner – to coordinate the transition.
✅ An executive sponsor – to provide leadership support and resources.
Having the right people involved ensures clear communication, accountability, and smooth collaboration throughout the process.
2. Evaluate Your Current QMS and Plan the Transition
Your implementation strategy depends on where you’re starting from. If you’re starting from scratch, focus on defining the essential QMS processes like:
📌 Document control
📌 Training and competency management
📌 Risk management and CAPA
If you’re migrating from a legacy system, make data transition a priority by:
🔍 Auditing current documentation to identify gaps.
🔍 Determining which processes need improvement or replacement.
🔍 Deciding on an approach – gradual migration or a full transition.
Taking the time to assess your current system helps avoid bottlenecks and inefficiencies down the road.
3. Focus on High-Priority Documents and Processes
To avoid delays and confusion, start with the most critical documents that your team needs for day-to-day operations. Categorizing your documents helps ensure a smooth transition:
📍 Immediate use: Work instructions, training records, and risk management files.
📍 Secondary priority: Regulatory compliance records and older quality reports.
📍 Archive or obsolete: Outdated CAPA records and non-relevant documents.
By organizing your documents this way, your team can start using the e-QMS effectively from day one without being overwhelmed.
4. Monitor, Measure, and Continuously Improve
Implementing an e-QMS isn’t just about getting it up and running—it’s about making sure it continues to add value. To maximize its effectiveness, you should:
✔️ Conduct regular internal audits to spot gaps and inefficiencies.
✔️ Hold management reviews to refine processes and improve compliance.
✔️ Provide ongoing training to ensure your team understands how to use the system effectively.
By embedding a culture of quality and continuous improvement, you’ll get the most out of your e-QMS and ensure long-term success.
Let’s Make Your e-QMS Implementation Smooth and Stress-Free!
Let Operon Strategist Help You Simplify Your e-QMS Implementation
At Operon Strategist, we specialize in medical device regulatory consulting and turnkey project management. Our experts can help you implement a robust e–QMS that meets regulatory requirements while optimizing your processes. Whether you need guidance on compliance, documentation, or process optimization, we’ve got you covered!