Overview - 6 Critical Regulations Shaping Medical Device Compliance
In the rapidly evolving world of medical device development, regulatory compliance is the bedrock of success. Identifying and adhering to all applicable regulations ensures safety, efficacy, and market acceptance. However, the complexity of regulations can be overwhelming, especially with the introduction of stringent frameworks like the MDR (Regulation (EU) 2017/745) and IVDR (Regulation (EU) 2017/746).
While MDR and IVDR provide extensive catalogs of device requirements and procedures, they represent only part of the compliance landscape. Many manufacturers fall into the trap of believing these regulations encompass all their obligations, overlooking additional critical requirements. This blog highlights six often-missed regulations and directives essential for achieving full compliance.
Looking For a Medical Device Regulatory Consultant?
Let’s have a word about your next project
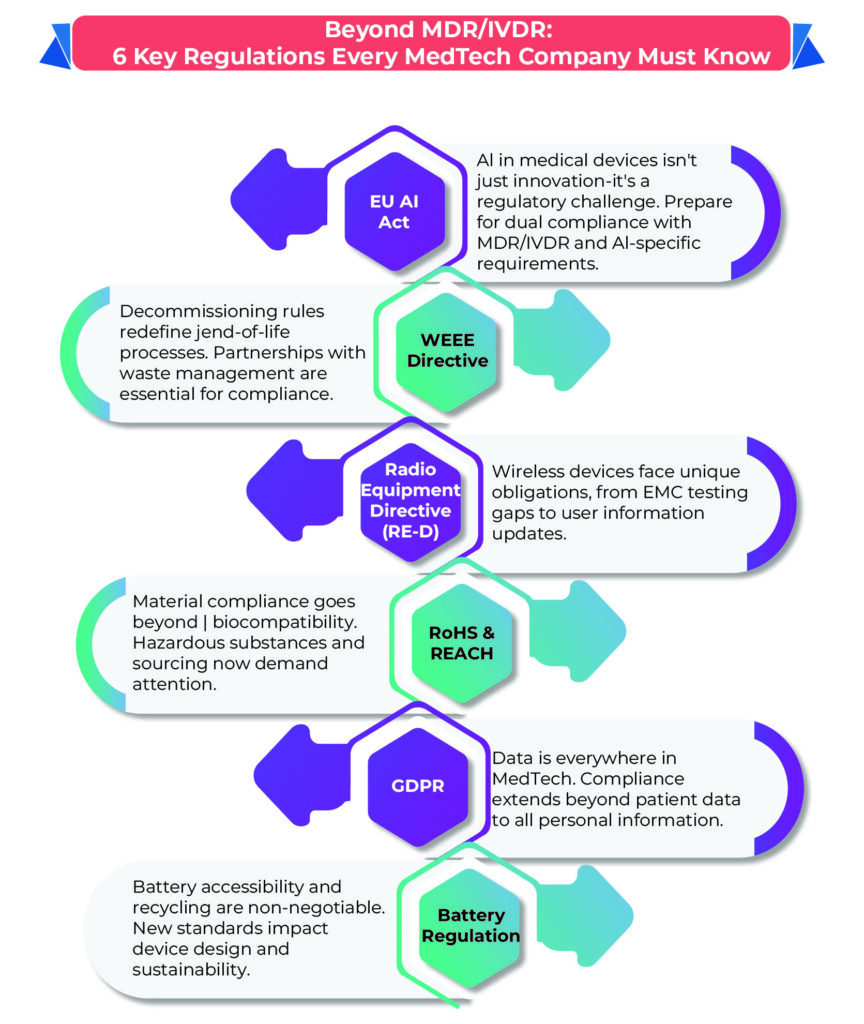
Six Overlooked Regulations Every Medical Device Manufacturer Must Know
- EU AI Act (Proposal COM/2021/206)
If your medical device incorporates artificial intelligence, the EU AI Act is a game-changer.
Key Implications:
- Introduces a certification framework beyond MDR/IVDR.
- Enforces overlapping requirements, making gap analysis indispensable.
A thorough understanding of this act ensures your AI-driven devices meet both functional and ethical standards.
- Radio Equipment Directive (2014/53/EU)
Devices with wireless communication, such as Bluetooth-enabled tools, fall under this directive.
Key Implications:
- Goes beyond MDR’s EMC testing requirements.
- Mandates additional instructions for use (IFU), including wireless frequency specifications.
- General Data Protection Regulation (Regulation (EU) 2016/679)
GDPR is crucial for devices interacting with personal data, even non-sensitive or non-health-related data.
Key Implications:
- Covers comprehensive data privacy requirements.
- Non-compliance can lead to hefty fines and reputational damage.
- Battery Regulation (Proposal COM/2020/798)
Medical devices with batteries—rechargeable or disposable—must adhere to this proposed regulation.
Key Implications:
- Ensures user access to batteries for removal or replacement.
- Enforces labeling and recycling compliance.
- RoHS (Directive 2011/65/EU) and REACH (Regulation (EC) No 1907/2006)
These regulations limit hazardous substances in device materials, ensuring environmental and user safety.
Key Implications:
- Material biocompatibility doesn’t guarantee compliance.
- Early material selection processes must align with these directives.
- WEEE (Directive 2012/19/EU)
This directive governs the decommissioning and disposal of electrical devices, including implantable and potentially infectious devices.
Key Implications:
- Requires agreements with waste management organizations.
- Includes specific exemptions, adding complexity to compliance.
Ready to Simplify Compliance?
Role of Operon Strategist in Navigating Regulatory Compliance
Operon Strategist is a leading medical device regulatory consulting company dedicated to simplifying the complex world of compliance. From initial product design to market entry, we provide end-to-end guidance tailored to your needs.
Why Choose Operon Strategist?
- Expertise Across Regulations: We ensure your compliance not just with MDR/IVDR but also with overlooked directives like WEEE, GDPR, and RoHS.
- Customized Solutions: Our team conducts gap analyses, develops regulatory strategies, and provides training to keep your organization audit ready.
- Global Reach: With a deep understanding of international regulations, we help you expand seamlessly into global markets.
Operon Strategist plays a key role in facilitating the export of medical devices from India to Russia by ensuring regulatory compliance. Specializing in global medical device regulations, Operon guides Indian manufacturers through the complexities of Russian import registration and certifications.
Their services cover regulatory approvals like FDA 510(k), European CE marking, and CDSCO registration, while also supporting the setup of compliant manufacturing facilities. Operon’s expertise in design, risk management, and technical documentation ensures that Indian exporters meet Russian standards, boosting trade relations between the two countries.