CDSCO Classification for Medical Devices and IVDs – Overview
The classification of medical devices by the Central Drugs Standard Control Organization (CDSCO) is governed by regulatory approvals and registrations issued under the Drugs Controller General of India (DCGI). In India, every medical device must comply with a regulatory framework based on the Drugs and Cosmetics Act, 1940, and the Drugs and Cosmetics Rules, 1945. The CDSCO categorizes medical devices into different risk-based classes, ensuring appropriate regulatory control based on potential risk to patient safety.
Additionally, both the CDSCO and respective State Licensing Authorities are responsible for granting licenses for medical devices across various categories, including items such as blood collection tubes, intravenous (I.V.) sets, and in vitro diagnostic (IVD) products.
Get Your Medical Device Classified by Experts
Share Your Product Details Below
CDSCO Registration
CDSCO (Central Drugs Standard Control Organization) is the national regulatory body for Indian medical devices, and pharmaceuticals and serves parallel functions to the European Medicines Agency of the European Union, the FDA (Food and Drug Administration) of the United States, and the medications and healthcare products Regulatory Agency of the United Kingdom.
It is important to have a CDSCO license for the medical device manufacturers and medical devices seller according to the Indian regulatory body. Operon Strategist will assist in getting a CDSCO Manufacturing license and CDSCO Import License as a medical device regulatory consultancy firm.
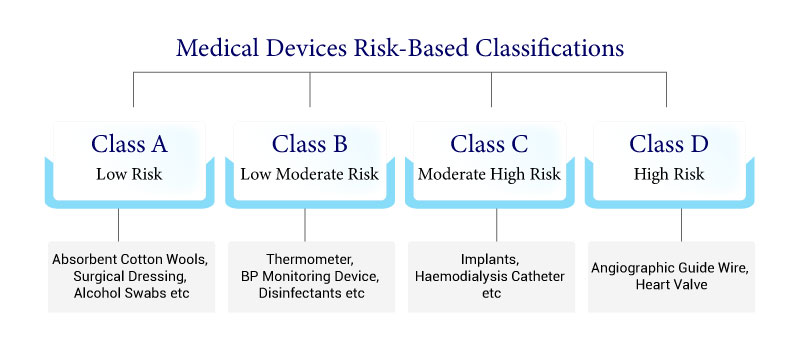
Medical devices are classified into four classes (A, B, C, and D) based on the risk of their intended usage. Find out more about it.
Operon Strategist Will Assist With Classifying and Registering Your Medical Product
CDSCO Classification for Medical Devices Risk-based Classifications
Medical Devices are generally based on risks; the actual risk-based classification of the medical device depends upon its intended use and purpose. CDSCO classification for medical devices has a larger group of devices, such as cannulas and stents in more specific subgroups.
Medical Devices and IVD are classified into four categories, depending upon the indications for use and risk level of the device (New Medical Device Rules 2018)
Four classes (Class A, B, C, and D) have been set up under the new system, where Class A and B present the least risk and Class C and D devices present higher risks to patients.
CDSCO is updating the Medical Device Classification regularly. Find all the newly notified device classifications here. Clarity On Classification Of Medical Devices
For example,
- An elastic bandage or a mechanical barrier used for pressure or ingestion of exudates for wounds that have not ruptured the dermis and can be healed by essential expectation would be classified as a Class A device.
- A case of a Class B medical device is contact lens points though a blood sack that doesn’t consolidate a medicinal product would be viewed as a Class C device.
- Lastly, a transient-utilize surgically obtrusive medical device expected to be utilized explicitly in direct contact with the central nervous system or for the diagnosis, checking, or adjustment of a heart defect or central circulatory framework condition through direct contact with body parts would be a case of a Class D device.
Read Here About Examples of Medical Devices
as per Classification of CDSCO, FDA and CE
These risk classifications will permit Indian medical device market registrants and their in-country delegates to improve the thought of issues, for example, cost, clinical information prerequisites, and import permitting authority as they market their products in India. The new guideline will adjust the utilization of medical device rules with International Standards to smoothen the manufacturing and importing of medical devices.
As medical device regulatory consultants, we help manufacturer classify their devices as per CDSCO guidelines and provide assistance in the CDSCO registration process for your medical devices. Click here to get assistance with CDSCO registration of medical devices.
Also, read Medical Device Classification EU MDR and FDA Medical Device Classification here.
FAQs
[rank_math_rich_snippet id=”s-2b59504b-b056-40f4-98c3-f298da41510d”]