Introduction: Why Medical Device Quality Assurance Matters
In today’s rapidly evolving healthcare industry, the role of medical devices is more critical than ever. From basic diagnostic tools to life-sustaining equipment, these devices directly impact patient health and outcomes. To maintain safety, performance, and regulatory compliance, Medical Device Quality Assurance (QA) plays a vital role across the entire product lifecycle—from design to post-market surveillance.
Whether you’re a new manufacturer or an established brand aiming to expand into regulated markets, understanding and implementing a robust QA system is key to product success. In this blog, we explore the essential elements of medical device QA, its importance, and how Operon Strategist helps ensure compliance and quality objectives are achieved efficiently.
Looking For a Medical Device Regulatory Consultant?
Let’s have a word about your next project
What Is Medical Device Quality Assurance?
Medical device quality assurance refers to a comprehensive system of processes and procedures that ensure a medical device is consistently produced and controlled to meet the quality standards required by regulatory authorities. The goal is to ensure product safety, reliability, and performance across its lifecycle.
QA is distinct from quality control (QC); while QC focuses on identifying defects in finished products, QA is a proactive system that aims to prevent quality issues during every phase of product development and manufacturing.
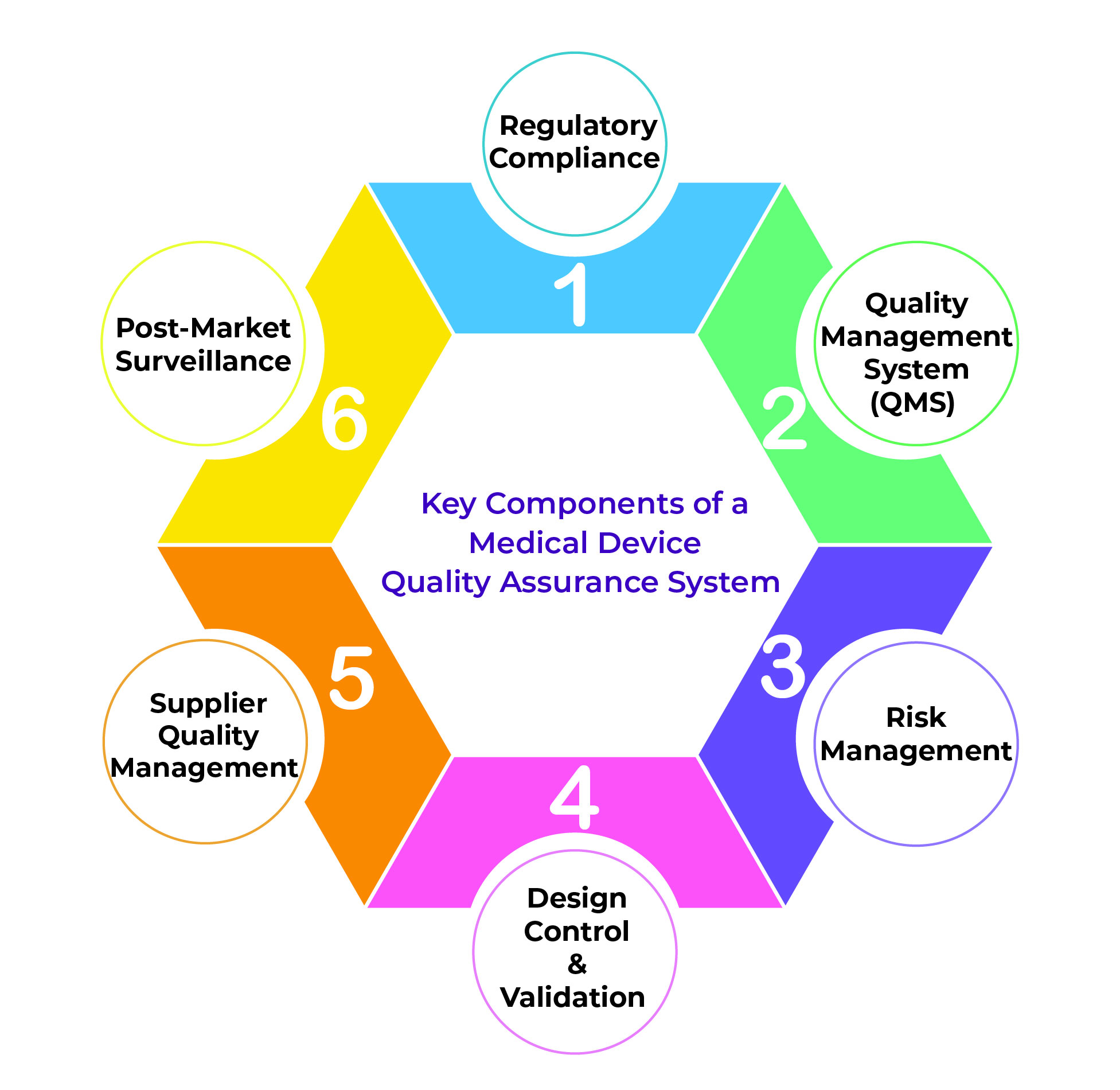
Key Components of a Medical Device Quality Assurance System
Here are the core elements that define an effective QA system for medical devices:
✅ Regulatory Compliance
Compliance with global regulatory standards like FDA 21 CFR Part 820, ISO 13485:2016, and EU MDR 2017/745 is non-negotiable. These standards provide the framework for ensuring medical device safety and performance.
✅ Quality Management System (QMS)
QMSrovides structured documentation and control of all processes, ensuring consistency and continual improvement. It includes SOPs for design control, validation, risk management, and CAPA (Corrective and Preventive Action).
✅ Risk Management
Identifying potential risks early in the development process through ISO 14971-compliant frameworks helps in minimizing adverse effects. Effective risk management ensures device safety and patient protection.
✅ Design Control & Validation
Design inputs, outputs, verification, and validation must be documented to prove that the device meets user needs and intended use. This ensures the device performs consistently across both batches and use cases.
✅ Supplier Quality Management
Suppliers play a critical role in device safety. Ensuring that all external vendors comply with your Quality Management System (QMS) standards is essential to avoid component failures or compliance breaches.
Continuous monitoring of devices after-marketrelease allows for quick response to adverse events or product feedback, supporting proactive quality improvements.
Importance of Medical Device QA in a Global Market
Implementing a strong QA system has wide-ranging benefits:
- Regulatory Approval: Gain access to regulated markets like the US, EU, and Gulf Cooperation Council (GCC) countries.
- Patient Safety: Minimize risks of malfunction and adverse events.
- Product Reliability: Ensure consistent product performance, earning user trust.
- Brand Reputation: Reduce recalls and penalties to enhance your credibility.
- Business Growth: Facilitate easier global expansion with compliance-ready documentation and systems.
For Expert QA System Implementation and Regulatory Support.
Benefits of Partnering with Operon Strategist for QA Implementation
Navigating the regulatory maze can be challenging without the right guidance. Operon Strategist, a leading medical device regulatory consulting firm, offers tailored solutions to implement or optimize your quality assurance (QA) systems. Here’s how we help:
- Development and implementation of ISO 13485 QMS
- Support for FDA 510(k), CE Marking, and CDSCO registrations
- Conducting internal audits and gap analysis
- Supplier audits and vendor compliance management
- Assistance with CAPA, risk management, and process validation
Looking to streamline your QA processes and meet global regulatory standards?
Contact Operon Strategist to build your robust Medical Device Quality Assurance system today!