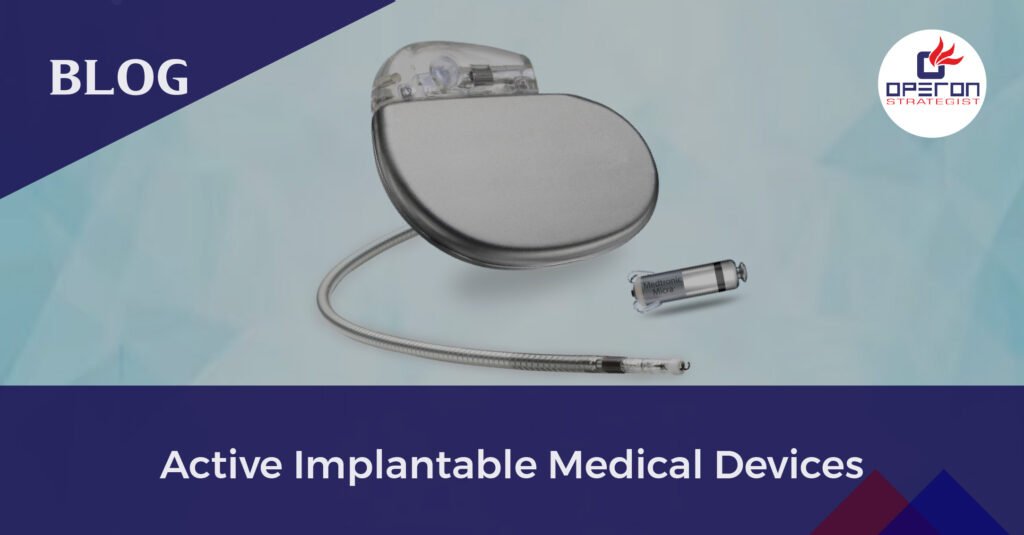
Active Implantable Medical Device (AIMD): Transforming Healthcare with Innovation
An active implantable medical device (AIMD) is a type of medical device that is designed to be implanted into the body for a long period of time. It uses electrical energy or a power source other than what is generated by the human body to perform its intended functions. AIMDs can be used for diagnostic or therapeutic purposes. These devices undergo rigorous testing and meet strict standards to ensure patient safety. They are carefully regulated to ensure their reliability and compatibility with the body. AIMDs play a crucial role in improving medical treatments and enhancing patient well-being.
Looking for Medical Device Regulatory Consultant?
Let’s have a word about your project
Examples of Active Implantable Medical Devices (AIMDs)
AIMDs include devices that help monitor and treat chronic conditions. Some common examples are:
- Pacemakers – Devices that regulate the heart’s rhythm, ensuring proper cardiac function.
- Blood Pressure Sensors – Continuous monitoring of blood pressure to manage hypertension.
- Cochlear Implants – Devices that restore hearing by bypassing damaged parts of the ear.
Importance of Active Implantable Medical Devices in Healthcare
AIMDs are crucial for modern healthcare due to their ability to provide continuous monitoring and long-term therapeutic benefits:
- Continuous Monitoring: AIMDs collect real-time data over an extended period, giving healthcare professionals a more comprehensive understanding of a patient’s condition. This allows for better diagnosis and management, particularly for intermittent or sporadic conditions.
- Therapeutic Benefits: Devices like pacemakers provide ongoing therapy that can improve the quality of life for patients with chronic conditions, such as heart disease, while also saving lives in critical situations.
By offering continuous monitoring and long-term care, AIMDs enable doctors to make informed decisions and deliver better, more targeted treatments for patients.
Manufacturing Process of Active Implantable Medical Devices
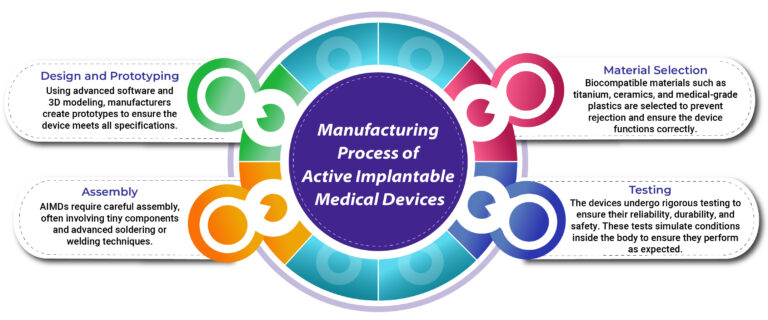
The manufacturing process for AIMDs is intricate and requires a high level of precision. These devices must be designed with materials that are biocompatible, durable, and capable of withstanding the harsh conditions within the body. The process includes:
- Design and Prototyping: Using advanced software and 3D modeling, manufacturers create prototypes to ensure the device meets all specifications.
- Material Selection: Biocompatible materials such as titanium, ceramics, and medical-grade plastics are selected to prevent rejection and ensure the device functions correctly.
- Assembly: AIMDs require careful assembly, often involving tiny components and advanced soldering or welding techniques.
- Testing: The devices undergo rigorous testing to ensure their reliability, durability, and safety. These tests simulate conditions inside the body to ensure they perform as expected.
Need More Clarity on Active Implantable Medical Devices?
Regulatory Pathway for Active Implantable Medical Devices
AIMDs are highly regulated due to their critical role in patient health. The regulatory pathway for these devices involves multiple steps:
- Pre-market Approval: Manufacturers must submit clinical data and testing results to regulatory bodies like the FDA (in the U.S.), CE (in Europe), and CDSCO (in India) to prove the device’s safety and efficacy.
- Quality System Regulations (QSR): Manufacturers must comply with regulations such as 21 CFR Part 820 (FDA) to ensure that the manufacturing process maintains high standards for safety and quality.
- Post-market Surveillance: After the device is approved, ongoing monitoring ensures it remains safe for use, with any adverse effects reported to regulatory bodies.
How Operon Strategist Can Help
Navigating the complex regulatory and manufacturing requirements for active implantable medical devices can be challenging. At Operon Strategist, we specialize in providing comprehensive support for AIMD projects. Whether you require regulatory guidance, manufacturing support, or assistance with the registration process, our team of experts is ready to help. Contact us today to learn more about how we can support your active implantable medical device projects.
FAQs:
Types of active implantable devices include pacemakers, implantable defibrillators, and neurostimulators.
An example of an implantable device is an artificial joint, breast implant, contraceptive intra-uterine device (IUD), or hardware for bone, muscle, and joint fusion.
Implant devices designed to regulate or monitor heart rhythms are known as cardiac implantable electronic devices, including pacemakers, implantable cardioverter defibrillators (ICDs), biventricular pacemakers, and cardiac loop recorders.
Implantable medical devices are classified as Class III devices, encompassing those that sustain or support life, are surgically implanted, or present a potential unreasonable risk of illness or injury. Examples include pacemakers and implanted prosthetics.