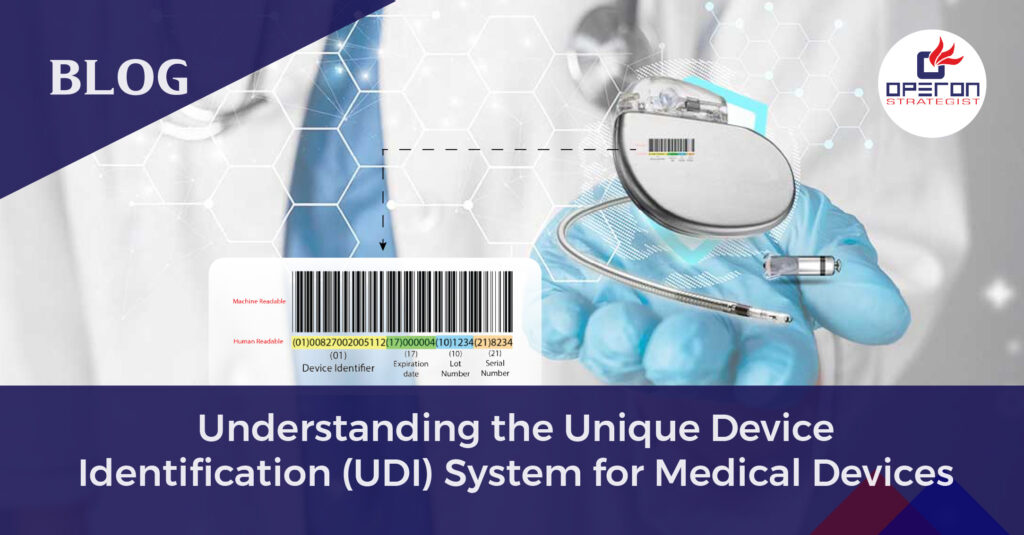
Understanding the Unique Device Identification (UDI) System for Medical Devices
UDI – Unique Device Identification Medical device regulations were implemented in the European Union in 2021 to make the medical device safer, more effective, and easy to trace in case something goes wrong. With the technological advancement, it was the main concern for the EU-MDR to trace those devices that either shows failure or adverse […]
Understanding the Unique Device Identification (UDI) System for Medical Devices Read More »