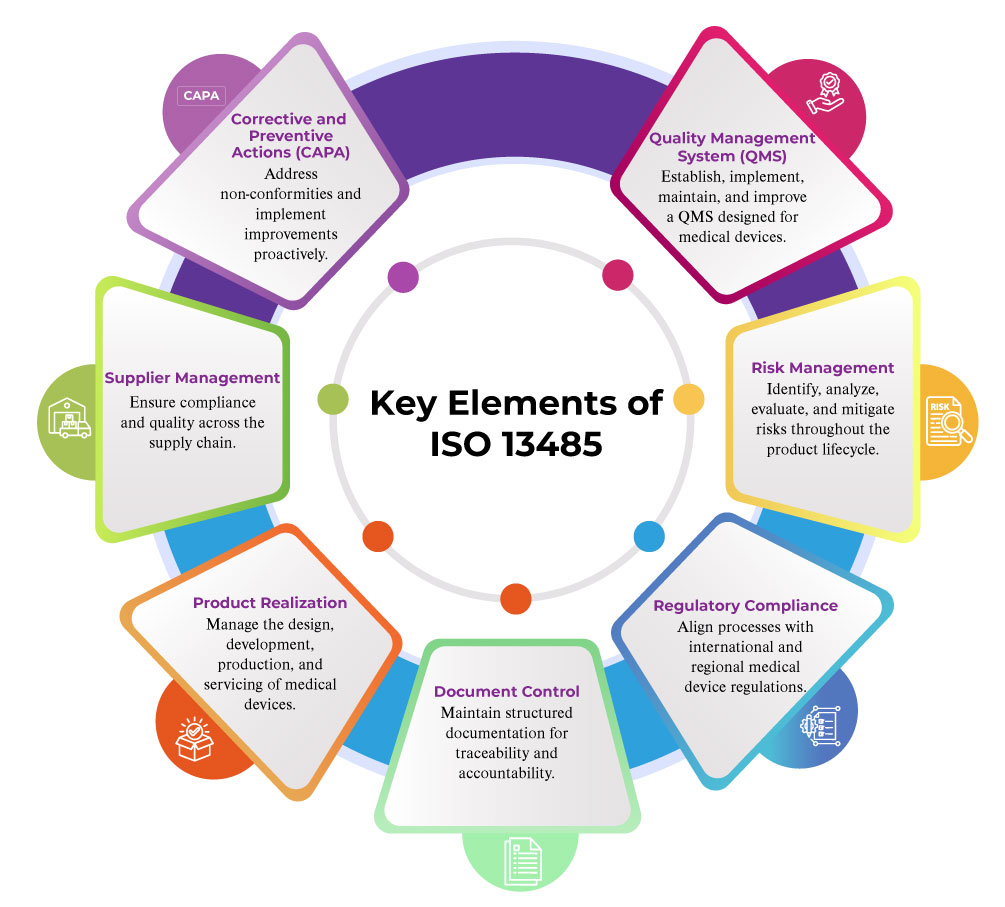
ISO 13485 is the internationally recognized standard for quality management systems (QMS) in the medical device industry. If you’re a MedTech professional—whether a manufacturer, supplier, or service provider—understanding and implementing ISO 13485 is crucial for ensuring product safety, meeting regulatory requirements, and expanding into global markets. In this blog, we break down ISO 13485 in a simple, human-centered way to help you navigate its importance and implementation.
Who Needs ISO 13485?
ISO 13485 is essential for various stakeholders in the medical device industry, including:
- Medical Device Manufacturers – Ensures compliance with regulatory authorities like the FDA and European MDR.
Looking For a Medical Device Regulatory Consultant?
Let’s have a word about your next project
- Suppliers & Vendors – Required to demonstrate adherence to quality and safety standards in the supply chain.
- Distributors – Helps maintain credibility and regulatory compliance when selling medical devices globally.
- Regulatory Consultants – Essential knowledge for guiding companies through certification and compliance processes.
- Testing & Calibration Labs – Ensures accurate, standardized testing for medical devices.
- Healthcare Facilities – Helpful for organizations that handle medical devices to ensure high-quality patient care.
If you’re involved in the lifecycle of a medical device, understanding and applying ISO 13485 can benefit your business and professional credibility.
Why is ISO 13485 Important for MedTech Professionals?
- Regulatory Approval: Compliance with ISO 13485 is often required for CE marking, FDA approvals, and other global certifications.
- Market Access: Many international markets require adherence to ISO 13485 as a prerequisite for entry.
- Product Quality and Safety: Ensures that medical devices meet stringent quality and safety standards.
- Operational Efficiency: Streamlines processes, reducing errors and inefficiencies.
- Competitive Advantage: Demonstrates a commitment to high standards, building trust with stakeholders.
Steps to Achieve ISO 13485 Certification
- Gap Analysis – Assess current processes against ISO 13485 requirements.
- QMS Implementation – Develop and integrate necessary policies and procedures.
- Training and Awareness – Educate employees on compliance and quality requirements.
- Internal Audits – Conduct audits to identify non-conformities and corrective actions.
- Certification Audit – Undergo an assessment by a notified body to achieve certification.
Common Challenges and How to Overcome Them
- Complex Documentation: Utilize digital tools for efficient document management.
- Regulatory Changes: Stay updated with evolving medical device regulations.
- Resource Constraints: Leverage expert consultants to guide implementation.
- Supplier Compliance: Establish strong supplier agreements and conduct regular audits.
How Operon Strategist Can Help?
At Operon Strategist, we specialize in guiding medical device companies through the ISO 13485 certification process. Our team of experts provides:
- Gap analysis and compliance assessments
- End-to-end QMS implementation
- Regulatory documentation support
- Internal audits and Training
- Assistance with certification audits
We help businesses streamline their quality management processes and ensure successful ISO 13485 certification, making compliance stress-free and efficient.
Frequently Asked Questions (FAQs)
No, but many regulatory authorities require it as part of their approval process. Compliance enhances credibility and global market access.
The timeline varies based on company size, existing processes, and regulatory preparedness. It typically takes 6-12 months.
If non-conformities are found, corrective actions must be taken before certification is granted. A follow-up audit may be required.
Yes, even startups and small businesses can implement ISO 13485 with the right strategy and expert guidance.