Identifying the Challenges in BIS Certification for Medical Devices
Getting BIS certification for medical devices can feel like a long and complex journey, but it’s a crucial step if you want to enter the Indian market. This certification ensures your product meets safety, quality, and performance standards. However, manufacturers often face hurdles along the way. In this blog, we’ll walk through the key challenges and share practical solutions to help simplify the process.
Medical Device Standards for BIS Certification
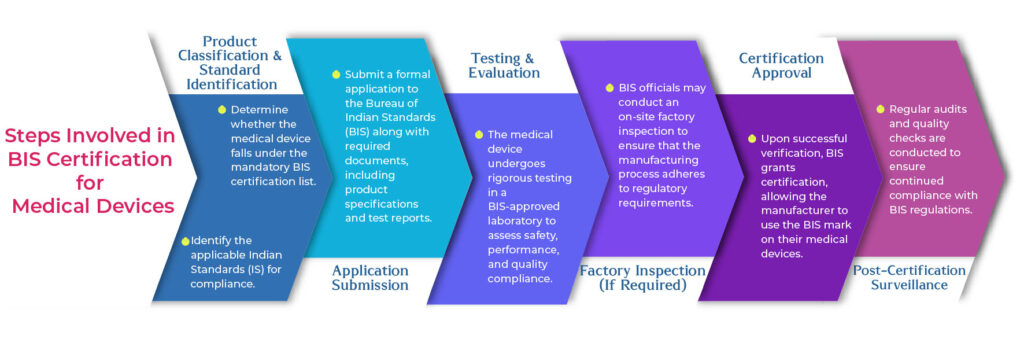
BIS certification, mandated under the Medical Devices Rules, 2017, ensures that medical devices comply with Indian Standards (IS).
Looking For a Medical Device Regulatory Consultant?
Let’s have a word about your next project
The process involves product testing, meeting compliance requirements, and getting approval from BIS authorities. The applicable standards for medical devices include:
- IS 23485 – Quality management system (QMS) requirements and fundamental principles of safety and performance for medical devices.
- IS 15579 – Infusion pumps
- IS 7372 – Syringes
- IS 13450 – Electromedical equipment
- IS 16098 – Dialyzers
- IS 16288 – Blood glucose monitoring systems
- IS 15185 – Cardiac pacemakers
Ensuring compliance with these standards is essential for the safety and effectiveness of medical devices used in healthcare settings.
Key Challenges in BIS Certification for Medical Devices
- Navigating Complex Regulations
- The BIS certification process can be overwhelming, especially for those new to the Indian regulatory landscape.
- Frequent updates to BIS guidelines can create uncertainty and slow things down.
How to Overcome It:
- Keep yourself updated with the latest BIS regulations by regularly checking official notifications.
- Work with an experienced BIS consultant who understands the process and can guide you through every step.
- Meeting Stringent Product Testing Requirements
- Medical devices must go through extensive testing in BIS-approved labs, which can take time and effort.
- Limited testing facilities can lead to delays in obtaining reports.
How to Overcome It:
- Plan ahead and schedule testing early to avoid last-minute delays.
- Partner with BIS-approved testing labs to ensure your product meets all required standards.
- Managing the Documentation Process
- The paperwork involved in BIS certification can be overwhelming, requiring technical documents, test reports, and compliance statements.
- Missing or incorrect documentation can lead to application rejection or additional delays.
How to Overcome It:
- Maintain a checklist of all required documents and double-check everything before submission.
- Get help from a BIS consultant to make sure your paperwork is in order.
- Handling the Costs of Certification
- The BIS certification process involves application fees, license fee, marking fee, testing charges, inspection charges and renewal fee, which can be expensive.
- For startups and small manufacturers, these costs can be a big challenge.
How to Overcome It:
- Budget ahead and explore financial support options to manage costs effectively.
- Focus on getting BIS certification for high-demand products first to maximize your investment.
- Maintaining Compliance for Renewals and Audits
- BIS certification is not a one-time process—it needs periodic renewals and compliance with surveillance inspections.
- Failing to meet requirements can lead to certification suspension or cancellation.
How to Overcome It:
- Set up a dedicated team to monitor regulatory updates and maintain compliance records.
- Conduct internal audits regularly to ensure you’re always prepared for official inspections.
Penalties for Non-Compliance with BIS Certification
Failing to obtain BIS certification for medical devices can lead to serious legal and financial consequences. Selling non-certified medical devices in India is a violation of the Bureau of Indian Standards Act, which can result in:
- Legal Action – Non-compliant companies may face strict legal actions, including bans on product sales and market recalls.
- Heavy Fines – Companies found violating BIS regulations can be subjected to hefty fines, increasing operational costs significantly.
- Loss of Market Access – Without BIS certification, medical devices cannot be sold in India, leading to potential revenue losses and market exclusion.
- Reputation Damage – Regulatory non-compliance can harm a manufacturer’s credibility, making it difficult to regain trust from healthcare providers and consumers.
To avoid these consequences, manufacturers must ensure their medical devices meet all BIS requirements before entering the Indian market.
Operon Strategist’s Role in BIS Certification
Operon Strategist simplifies BIS certification for medical device manufacturers by offering expert regulatory support. Our team ensures compliance with BIS requirements, streamlining the entire certification process from consultation to documentation and product testing. We assist in preparing technical and regulatory documents, coordinating with BIS-approved labs, and optimizing certification costs without compromising compliance. Beyond certification, we provide ongoing support for renewals, audits, and regulatory updates. In addition to BIS certification, we offer consultation for CE marking, FDA 510(k) submissions, ISO compliance, and turnkey project support, helping manufacturers enter the Indian market smoothly and efficiently.
FAQs
It ensures compliance with Indian safety, quality, and performance standards under the Medical Devices Rules, 2017.
Common issues include complex regulations, strict testing, heavy documentation, high costs, and renewal compliance.
It typically takes 3 to 6 months, depending on testing, documentation, and regulatory approvals.
We provide consultation, documentation support, testing coordination, and compliance management for a hassle-free process.