Medical Device Single Audit Program (MDSAP) Consulting Services
Understanding the Medical Device Single Audit Program (MDSAP)
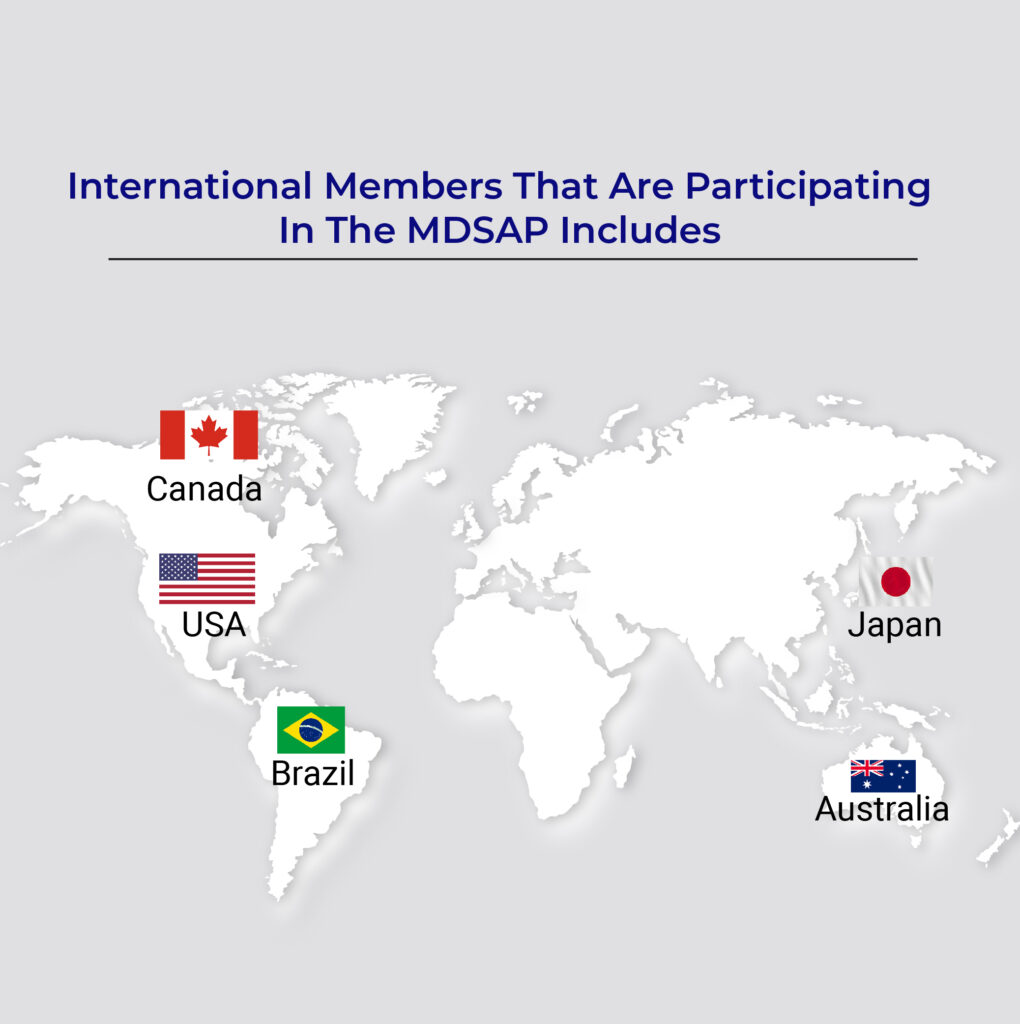
The Medical Device Single Audit Program (MDSAP) is a globally recognized initiative introduced to simplify and standardize the auditing process for medical device manufacturers. This program allows a single regulatory audit to satisfy the quality management system (QMS) requirements of multiple countries, including:
- USA: Compliance with the FDA’s 21 CFR Part 820.
- Canada: Adherence to the Medical Devices Regulations (CMDR).
- Brazil: Alignment with ANVISA requirements.
- Japan: Compliance with the Pharmaceuticals and Medical Devices Act (PMDA).
- Australia: Conformance with TGA regulatory standards.
By integrating these regulatory frameworks into one cohesive audit process, MDSAP reduces duplication and streamlines compliance efforts, saving both time and resources for medical device companies.
Let's Grow Your Business Together
How MDSAP Certification Benefits Medical Device Manufacturers
Achieving MDSAP certification provides several advantages:
- Eliminates the need for multiple regulatory audits.
- Strengthens your organization’s global credibility.
- Ensures compliance with international regulations, including the medical device single review program standards.
How we help: Operon Strategist ensures your MDSAP compliance by offering customized consulting services, including process evaluations, documentation support, and pre-audit preparation.
Learn more about how we assist in meeting compliance standards on our turnkey projects page.
Step-by-Step Guidance Through the MDSAP Certification Process
The MDSAP certification process involves the following steps:
- Training and Preparation: Understand the fundamentals of the Medical Device Single Audit Program (MDSAP training) and its scope.
- Gap Analysis: Identify compliance gaps in your quality management system (QMS) based on the MDSAP standard.
- Audit Support: Implement and monitor corrective actions for seamless certification.
Our experts ensure all regulatory documentation aligns with the requirements of the MDSAP certification process, preparing your team for successful audits.
Importance of MDSAP Auditor Certification and Training
Auditors are key to the Medical Device Single Audit Program (MDSAP) process. They evaluate your systems for compliance. Operon Strategist provides training for MDSAP auditor certification, focusing on:
- Key principles of the Medical Device Single Audit Program.
- Requirements outlined in the MDSAP standard.
- Practical insights to improve audit readiness.
Visit our validation documentation page to learn how we assist with audit readiness.
Operon Strategist’s Role in MDSAP Compliance
Operon Strategist offers a comprehensive suite of services to help you achieve MDSAP certification efficiently:
- Gap assessment and implementation support for the MDSAP certification process.
- Guidance on the medical device single review program and related documentation.
- Tailored training for your team on Medical Device Single Audit Program (MDSAP training).
- Detailed preparation for compliance with MDSAP requirements and regulatory standards
Explore our expertise on our regulatory approvals page.
Why Choose Operon Strategist for MDSAP Consulting?
Our experience in global regulatory frameworks makes Operon Strategist the ideal partner for achieving compliance with the Medical Device Single Audit Program (MDSAP). We provide:
- End-to-end consulting services.
- Comprehensive training for meeting MDSAP requirements.
- Expert support to align your QMS with the MDSAP standard.
Discover how we simplify certification processes for medical devices on our services page.
