CT scan machines have revolutionized medical imaging, providing clear, detailed 3D views of the body for accurate diagnosis and treatment. Unlike traditional X-rays, CT scanners capture multiple images from different angles, helping doctors detect complex conditions with precision.
At Operon Strategist, we guide manufacturers through every step of CT scan equipment manufacturing, from regulatory approvals to quality management and production setup. With our expertise, we ensure compliance with global standards, making the process smooth and efficient.
Looking for Consultant?
Let’s have word about your project
Understanding CT Scan Equipment Manufacturing
How It Works
CT scan machines use advanced imaging technology to create highly detailed, cross-sectional views of the body. As a patient lies on a motorized table, the scanner rotates and emits controlled X-ray beams, which pass through the body. High-sensitivity detectors capture these signals and send them to a computer, where powerful algorithms reconstruct the data into clear, 3D images. This allows doctors to analyze internal structures with incredible accuracy, making CT scans essential for diagnosis and treatment.
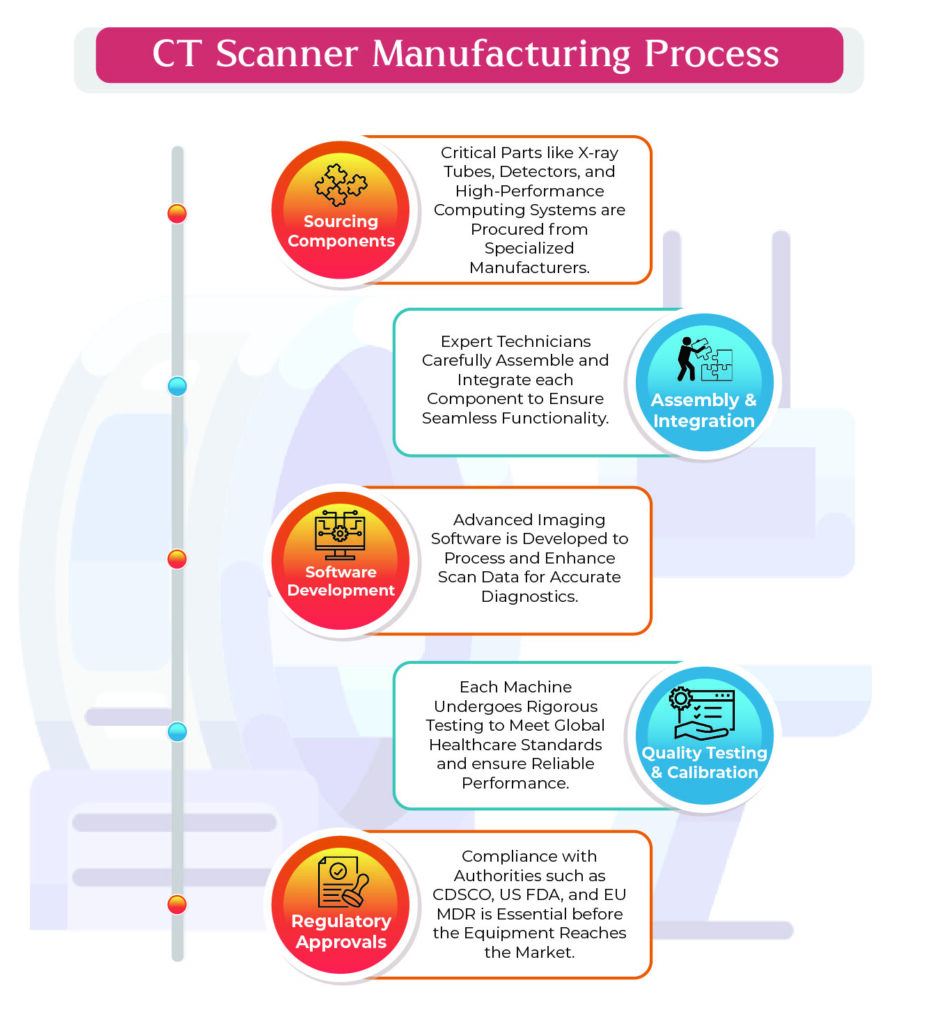
Key Components in CT Scan Equipment Manufacturing
- X-ray Tubes: Essential for producing the radiation beams that penetrate the body.
- Detectors: Capture X-ray signals and convert them into digital images.
- Rotating Gantry: Houses the X-ray tube and detectors, ensuring smooth scanning motion.
- Image Processing Software: Enhances captured images for medical analysis.
- Control Console: Allows radiologists to operate the machine and adjust settings.
- High-Performance Computing System: Processes data rapidly to produce real-time imaging.
Regulatory Compliance for CT Scan Equipment Manufacturing
Manufacturers must comply with stringent regulations to ensure product safety and efficacy. Some key regulatory bodies include:
1. India (CDSCO Compliance)
- CT scan equipment manufacturers must register with the Central Drugs Standard Control Organization (CDSCO) and obtain the required license as per MDR 2017.
- A CDSCO manufacturing consultant can assist in documentation, submission, and approval processes.
2. United States (FDA Compliance)
- The U.S. Food and Drug Administration (FDA) classifies CT scan equipment as a medical imaging device.
- Manufacturers may need 510(k) clearance or Premarket Approval (PMA) depending on the risk classification.
3. Other Global Markets
- European Union: Compliance with EU MDR (Medical Device Regulation).
- Saudi Arabia: Approval from the Saudi Food and Drug Authority (SFDA).
- United Kingdom: Compliance with the MHRA (Medicines and Healthcare products Regulatory Agency).
Challenges in CT Scan Equipment Manufacturing
- High Capital Investment: Manufacturing CT scanners requires advanced technology, skilled workforce, and significant financial resources.
- Sourcing Components: Key components like tubes, detectors, and magnets are often imported from OEMs.
- Precision Engineering: Achieving accurate imaging requires sophisticated design and calibration.
- Regulatory Hurdles: Navigating complex approval processes across multiple jurisdictions.
The Role of CT Scan Equipment in Modern Healthcare
- Neurology: Detects brain abnormalities and strokes.
- Oncology: Identifies tumors and guides treatment planning.
- Orthopedics: Assesses fractures and bone health.
- Cardiology: Evaluates heart conditions and vascular diseases.
Optimizing CT Scan Equipment Manufacturing for Efficiency
- Advanced Quality Control: Implementing automated inspection systems.
- Integration of AI: Enhancing imaging accuracy and diagnostic capabilities.
- Reducing Production Time: Using innovative manufacturing techniques to streamline assembly.
- Regulatory Compliance Assistance: Partnering with expert consultants for smooth approval processes.
Why Choose Operon Strategist?
At Operon Strategist, we understand the challenges of CT scan equipment manufacturing and are here to make the process smoother for you. From navigating complex regulatory approvals to setting up a fully compliant manufacturing plant, we provide expert guidance every step of the way. Our team helps you implement a strong Quality Management System (QMS) to ensure your products meet the highest industry standards. We also assist with compliance audits and fine-tuning your processes so you can focus on what matters most—delivering high-quality medical devices. With us, you can confidently bring your CT scan equipment to market.
FAQs
It uses X-ray beams and detectors to capture multiple images, creating detailed 3D scans for accurate diagnosis.
X-ray tubes, detectors, a patient table, and a computing system process images for precise results.
Manufacturers must comply with CDSCO, US FDA, and EU MDR for market approval.
Optimizing sourcing, assembly, quality control, and regulatory compliance ensures efficiency.