If you manufacture custom-made medical devices, navigating the European Union’s Medical Device Regulation (EU MDR 2017/745) is essential for compliance and continued market access. These devices are tailor-made to meet individual patient needs but must still meet strict EU safety, performance, and documentation requirements.
Gain a clear understanding of what qualifies as custom made medical devices under EU MDR, along with the key regulatory obligations, essential documentation, and effective compliance strategies manufacturers must follow to meet EU requirements.
What Is a Custom-Made Medical Device under MDR?
A custom-made medical device is specifically manufactured for an individual patient, based on a written prescription provided by a qualified medical professional. These devices are unique and not produced in series or sold off the shelf.
Looking For a Medical Device Regulatory Consultant?
Let’s have a word about your next project
Examples include:
- Dental prosthetics
- Patient-specific orthopedic implants
- Personalized hearing aids
Under EU MDR 2017/745, custom made medical devices are treated differently from standard CE-marked devices. Although they do not require CE marking, manufacturers must still ensure full compliance with applicable regulatory requirements.
MDR Provisions for Custom Made Medical Devices
The MDR defines custom made medical devices in Article 2(3). While exempt from CE marking, CMDs must adhere to critical safety, performance, and documentation expectations.
Key MDR Articles Relevant to CMDs:
- Article 10 – Outlines manufacturer obligations including risk management, quality systems, and safety.
- Annex XIII – Specifies requirements for the manufacturer’s statement and technical documentation.
Regulatory Requirements for Custom Made Medical Devices
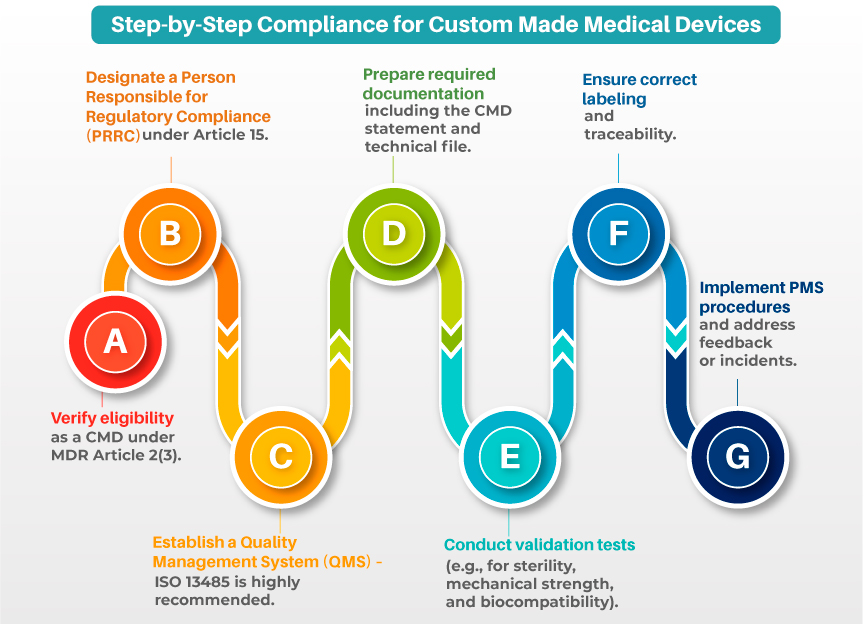
Even though CMDs are exempt from CE marking, manufacturers must complete several essential compliance steps:
- Written Prescription and Design Justification
Each device must be created from a written prescription specifying the design requirements based on the patient’s condition.
- Manufacturer’s Declaration
A formal statement must be issued confirming the device is intended solely for one individual patient, as per MDR Annex XIII.
- Technical Documentation
Your technical file for custom-made medical devices should include:
- Device description and intended use
- Design files and material specifications
- Risk analysis
- Validation of critical processes (e.g., sterilization, biocompatibility)
- General Safety and Performance Requirements (GSPR)
The device must meet the standards laid out in Annex I of the MDR, covering mechanical integrity, chemical safety, usability, and more.
- Labelling Requirements
Labels must include:
- The phrase “custom made medical device”
- Manufacturer’s name and address
- Name of prescribing healthcare professional
- Any warnings or precautions
- Post-Market Surveillance (PMS)
MDR mandates ongoing evaluation of real-world performance for custom-made medical devices to ensure ongoing safety and effectiveness.
Common Pitfalls in CMD Compliance
Manufacturers often underestimate the regulatory obligations for custom made medical devices. Key challenges include:
- Incomplete or missing technical documentation
- Improper labeling and omission of required declarations
- Neglecting post-market surveillance responsibilities
- Failing to assign a PRRC as required under MDR
- Overlooking clinical validation despite personalized design
How Operon Strategist Helps with CMD Compliance
At Operon Strategist, we support manufacturers of custom-made medical devices through every stage of EU MDR compliance. Our services include:
- CMD eligibility assessment
- Technical documentation preparation
- QMS setup aligned with ISO 13485
- Regulatory guidance for PRRC designation
- Validation strategy development
- Post-market surveillance planning
With our tailored support, manufacturers can reduce regulatory risks and streamline their path to EU market entry.
Avoid costly mistakes get custom device regulatory support today.
FAQ'S
What qualifies as a custom-made medical device under EU MDR?
A CMD is made specifically for one patient based on a prescription from a qualified healthcare provider. It is not manufactured in batches or for general sale.
Do custom-made medical devices require CE marking?
No. CMDs are exempt from CE marking but must meet all other applicable MDR requirements.
What documentation is required for custom-made medical devices?
Manufacturers must maintain a CMD statement, technical documentation, labeling, and post-market surveillance reports.




