Transforming Digital Dentistry with Precision
Dental scanners have revolutionized modern dentistry by providing highly accurate digital impressions of teeth, gums, and oral structures. Using advanced imaging technology, these scanners capture detailed 3D models that enhance precision in orthodontics, restorations, and treatment planning. As digital dentistry continues to evolve, dental scanners are redefining accuracy, efficiency, and patient comfort.
Explore more about Dental Implants Manufacturing
Looking For a Medical Device Regulatory Consultant?
The Manufacturing Process of Dental Scanners
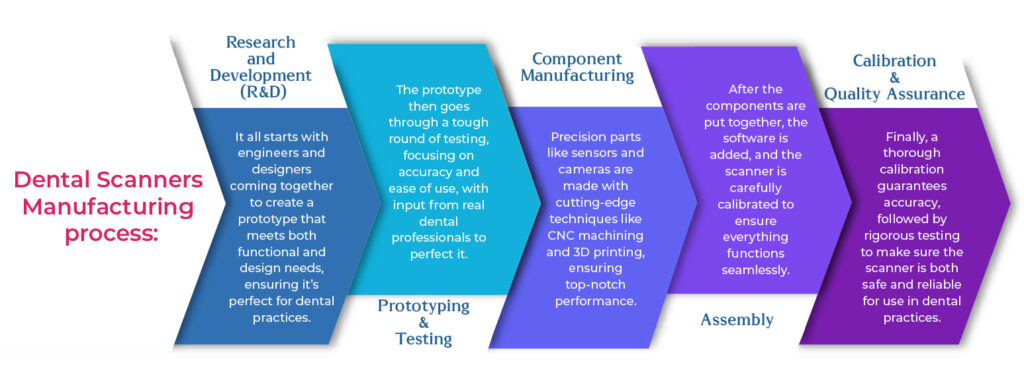
Creating a dental scanner is a meticulous process that combines cutting-edge technology with high precision. Every stage, from initial research to final assembly, ensures the highest quality and reliability.
1. Research and Development (R&D)
The journey begins with engineers and designers collaborating to develop a prototype that meets both functional and ergonomic needs. Extensive research ensures the scanner is practical, efficient, and suited for dental professionals.
2. Prototyping & Testing
Before mass production, a prototype is created and rigorously tested. Dental professionals provide feedback on ease of use, accuracy, and comfort, helping to refine the design for optimal performance.
3. Component Manufacturing
Key components like high-resolution sensors, advanced cameras, and precise scanning optics are manufactured using technologies like CNC machining and 3D printing. These ensure durability, accuracy, and seamless operation.
4. Assembly & Software Integration
Once the components are ready, they are assembled with precision. The scanner’s software, which processes and converts 3D scan data, is integrated to ensure smooth performance and user-friendly operation.
5. Calibration & Quality Assurance
Each scanner undergoes meticulous calibration to guarantee scanning precision. Rigorous quality checks, including performance and durability testing, ensure reliability before the scanners are distributed to dental clinics.
Obtain Your CDSCO Manufacturing License for Dental Scanners with Expert Guidance
Operon Strategist: Your Turnkey Partner in the Future of Dental Scanners
Dental scanners are revolutionizing modern dentistry, making treatments faster, more precise, and more comfortable for patients. As technology advances, these devices will continue to enhance efficiency, accuracy, and patient outcomes—leading the way in digital dentistry. However, bringing a dental scanner from concept to production requires expertise in manufacturing setup, process optimization, and quality assurance—and that’s where Operon Strategist steps in.
We provide end-to-end turnkey solutions, guiding manufacturers through every stage:
- Facility Design & Setup – Optimizing the manufacturing plant layout for efficiency and compliance.
- Cleanroom Setup & Validation – Ensuring a contamination-free production environment.
- Process & Machine Validation – Enhancing operational precision with validated workflows.
- Supply Chain & Vendor Management – Sourcing high-quality components for superior performance.
- Quality Control & Production Optimization – Implementing best practices for seamless manufacturing.
With Operon Strategist, manufacturers can focus on innovation while we handle the complexities of scaling production, optimising workflows, and ensuring top-tier quality. Whether you’re setting up a new facility or improving an existing one, we provide expert guidance and tailored turnkey solutions to bring your dental scanners successfully to market.
Ready to elevate your dental scanner manufacturing process? Contact Operon Strategist today!




