Medical Device Design and Development Services in Brazil
What Is Medical Device Design and Development?
Medical device design and development is a structured, innovation-driven process that transforms clinical needs into practical, safe, and market-ready healthcare solutions. It goes beyond shaping an idea; it ensures devices are effective, compliant with global standards such as ISO 13485, ANVISA, and FDA guidelines, and meet the needs of healthcare providers and patients.
Through careful planning, risk management, prototyping, and testing, medical device design and development help manufacturers create products that improve patient outcomes while complying with local and international regulatory requirements.

Key Pillars of Medical Device Design and Development
Market Demand: Identifying unmet healthcare needs and aligning device features with current clinical practices and trends in Brazil.
Clinical Impact: Defining how the device enhances diagnosis, treatment, or patient care.
User & Patient Safety: Incorporating risk management, usability engineering, and validation protocols to ensure safety throughout the product lifecycle.
Regulatory Compliance: Ensuring devices meet ANVISA, ISO 13485, FDA, and CE/UKCA standards for safe and legal market access.
From concept to commercial production, medical device design and development balance innovation, usability, and regulatory compliance to deliver reliable, market-ready solutions.
Let's Grow Your Business Together
Why Medical Device Design and Development Matters
Proper medical device design and development:
- Improves patient care and addresses unmet medical needs in Brazil.
- Reduces operational errors and enhances device usability.
- Ensures compliance with ISO 13485:2016 Clause 7.3, FDA 21 CFR Part 820.30, and ANVISA regulations.
- Supports innovation for next-generation healthcare solutions.
The Medical Device Design and Development Process Guide
During the design and development phase, Operon Strategist assists Brazilian medical device manufacturers in following a structured process to ensure full regulatory compliance. After analyzing a new medical device concept, the next critical step is the actual device design. This stage is pivotal, as a flawed design can lead to ineffective, unsafe, or non-compliant products. Implementing a robust design control system at this stage is essential to ensure that the final product meets user needs, clinical requirements, and market expectations.
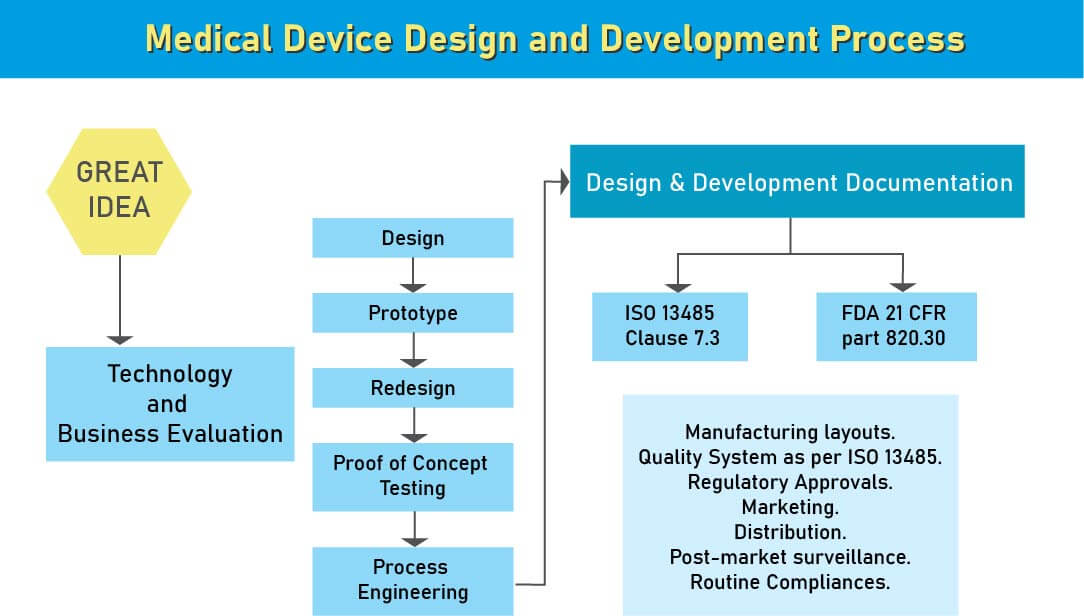
Design controls are logical, step-by-step procedures that verify the product is developed exactly as intended. Medical device development involves numerous components and complex interactions across various device types. A clear product definition, based on market analysis and user requirements, forms the foundation for successful design. Device classification, based on risk and regulatory guidelines, determines the level of design control required to enter the market. Compliance with both local (ANVISA) and international regulations is essential to ensure safety, quality, and usability.
Global standards, including ISO 13485, provide detailed guidance for medical device quality management. Regional or country-specific regulations in Brazil are aligned with these international standards, with minor adaptations to meet local requirements.
Medical device manufacturers must adhere to design control principles, as regulatory authorities such as ANVISA, FDA, and the European Commission require documented evidence that devices are safe and effective before market entry. Design control begins with design input development and approval, covering both product and manufacturing processes. It is a continuous, iterative process that extends beyond finalizing the design, influencing manufacturing practices and allowing for improvements based on user feedback or product performance analysis. This ongoing process ensures the final device is safe, effective, and fully aligned with market needs.
Design Control for Medical Devices
Design control is a structured regulatory approach ensuring medical devices are safe, effective, and compliant. For Class II and III devices, design control involves documented processes that demonstrate safety, intended use, and regulatory adherence throughout the development lifecycle.
Key steps include:
- Device classification and risk assessment
- Design input and output management
- Verification and validation
- Design transfer to manufacturing
- Continuous design improvement
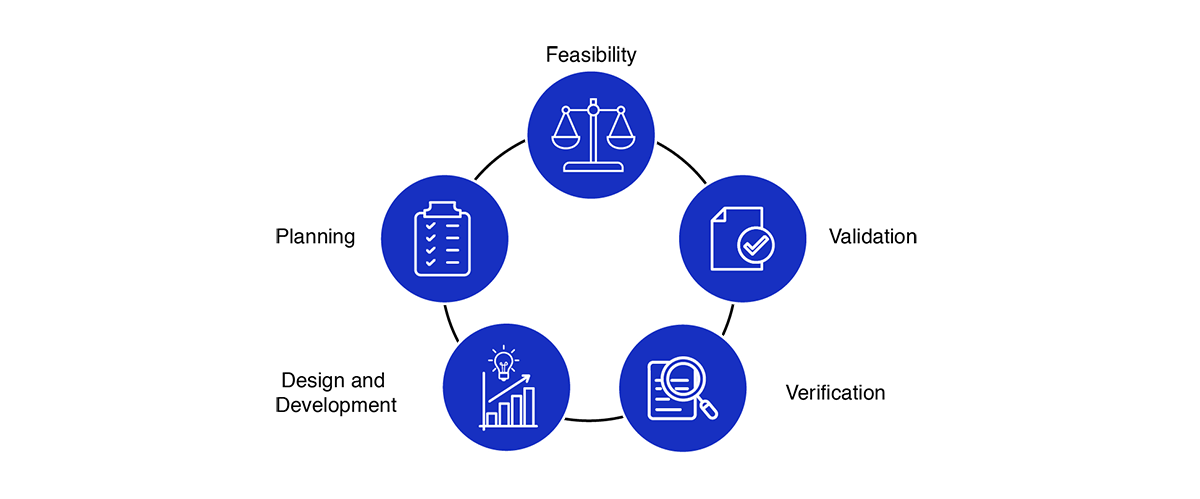
Medical Device Design Services & Product Development Process?
- Feasibility
- Planning
- Design and development
- Verification
- Validation
The Medical Device Design and Development Services Includes :
Combination Products – Drug + Device:
For combination products, such as prefilled syringes or topical applicators, manufacturers must carry out comprehensive design and development activities. This ensures that the device is both safe and effective. Operon Strategist follows a systematic, regulatory-compliant methodology to establish proper design and development processes, guaranteeing product quality and market readiness.
Medical Device Design Control
Once a new medical device concept is defined, the next critical step is the design phase. This stage is essential, as a flawed design can make a device unsafe, ineffective, or non-compliant with regulatory authorities such as ANVISA, FDA, or CE. At this stage, a robust design control system is implemented as part of the quality management process, ensuring that the final product meets user needs, regulatory requirements, and clinical expectations.
Get expert guidance on design, development, and regulatory compliance in Brazil.
How Operon Strategist Can Help
Our experts provide end-to-end consulting for medical device design and development in Brazil:
- Regulatory strategy & device classification
- Design input/output management
- Risk management and usability engineering
- Tool validation and QMS setup
- Staff training for regulatory compliance
We help bring innovative medical devices to market safely, efficiently, and in full regulatory compliance.
FAQ'S
What is medical device design and development?
It is the process of creating safe, effective medical devices, including planning, risk management, prototyping, testing, and regulatory compliance.
Why is design control important in medical device development?
Design control ensures devices are developed systematically, meet regulatory standards like ISO 13485 and FDA 21 CFR 820.30, and achieve market approval safely.
Are design controls required for medical device manufacturers in Brazil?
Yes, manufacturers must implement design controls to comply with ANVISA, ISO 13485, and international standards for market access.
How can Operon Strategist help with medical device design in Brazil?
We offer end-to-end consulting, from risk management and tool validation to QMS setup and regulatory compliance training.
What is the first step in developing a medical device in Brazil?
Identifying the medical need and defining user requirements, followed by device classification and design control implementation.