Medical Device Clean Room Design Consultant in Brazil
Importance of Clean Room Design for Medical Devices
Medical device clean room design plays a vital role in ensuring product quality, safety, and compliance with international regulations. A clean room is a controlled environment that limits dust, airborne particles, microbes, and chemical vapors, making it essential for medical device manufacturing in Brazil.
At Operon Strategist, we provide end-to-end clean room design and consultation services for medical device manufacturers, ensuring compliance with ANVISA, ISO 13485, and ISO 14644-1 standards.

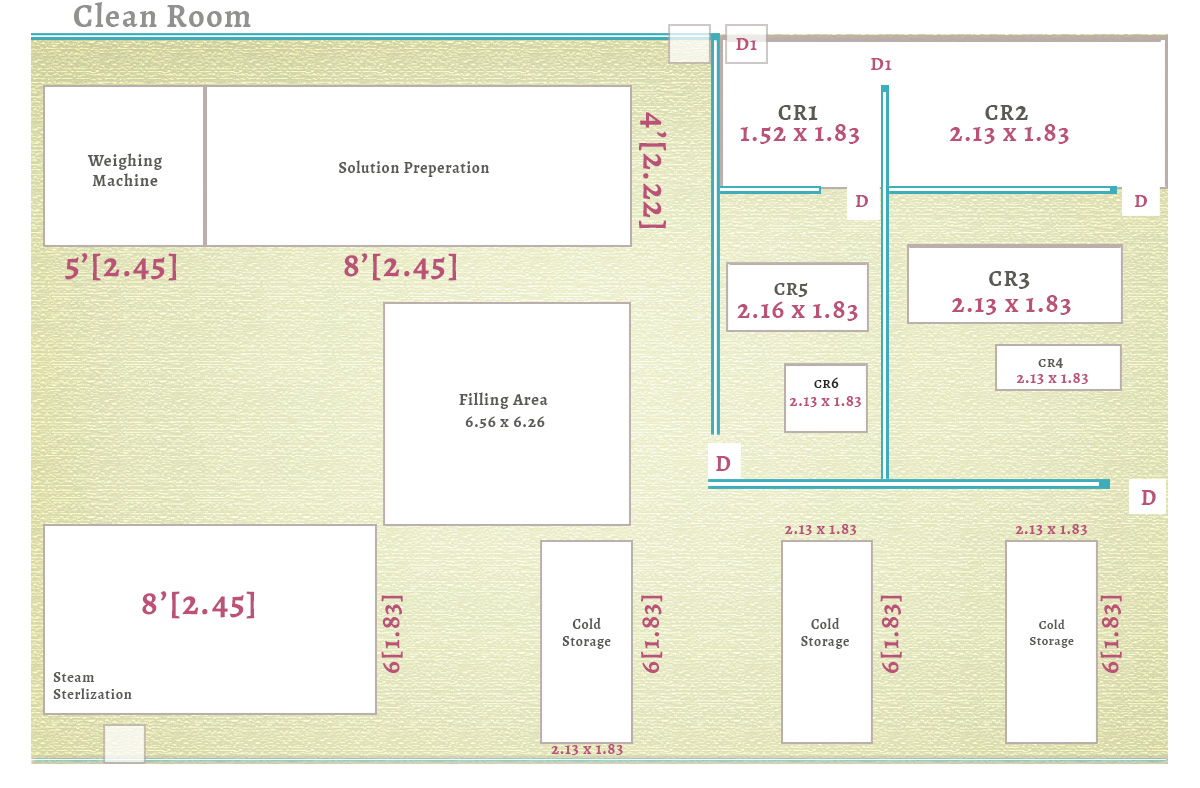
What is a Medical Device Clean Room?
A medical device clean room is a specially designed enclosed facility that controls environmental parameters such as air quality, humidity, pressure, and personnel access. This ensures that manufacturing takes place in a contamination-free environment, maintaining product safety and effectiveness.
Industries like medical devices, pharmaceuticals, diagnostics, and biotechnology rely heavily on clean rooms to meet regulatory requirements and achieve operational efficiency.
Why Do Medical Device Manufacturers Need Clean Rooms?
- To comply with ANVISA medical device regulations.
- To maintain sterile and controlled environments for device manufacturing.
- To meet ISO 13485 & ISO 14644-1 clean room standards.
- To reduce contamination risks and ensure patient safety.
- To enhance efficiency and achieve successful regulatory approvals.
Medical Device Clean Room Regulations in Brazil
For medical device manufacturers, compliance with ISO 13485 and ISO 14644-1 standards is crucial. These standards regulate clean room design, monitoring, and control systems to ensure product safety.
As a medical device regulatory consultant in Brazil, Operon Strategist helps implement QMS systems aligned with ANVISA and international requirements, ensuring clean rooms meet regulatory expectations.
Let's Grow Your Business Together
How Operon Strategist Helps in Medical Device Clean Room Design
Operon Strategist provides comprehensive consulting services for clean room design in Brazil, including:
- Medical device clean room design & validation as per ANVISA & ISO standards.
- Regulatory documentation support for compliance and audits.
- AutoCAD design services for clean room layouts and facility planning.
- Environmental control system design for air circulation, humidity, temperature, and pressure.
- Operational guidelines & training including gowning procedures, entry-exit protocols, and contamination prevention.
We ensure your clean room is not only regulatory-compliant but also efficient and sustainable.
Why Choose Operon Strategist?
- Expertise in Brazil ANVISA clean room regulatory compliance.
- Proven experience in completing clean room projects globally.
- End-to-end consulting from design conceptualization to validation and approval.
- Tailored solutions for medical device manufacturers, R&D labs, and healthcare facilities.
With years of experience in working with regulatory bodies worldwide, including ANVISA, SFDA, MHRA, and FDA, we ensure your facility meets all compliance requirements.
In Brief, Operon Strategist is a medical device regulatory consulting company that provides regulatory advisory & guidance to various manufacturers in the healthcare industry to ensure the strategic development of these manufacturers. We are one of the leading regulatory consultants working closely with various regulatory authorities like SFDA, EDA, and MHRA. To implement a proper QMS system for your manufacturing unit or design a clean room you can freely contact us. We also provide medical device consultation for India, Saudi Arabia, the USA, the UK, South Africa, Oman, Iran & Egypt. Feel free to contact us.
FAQs
Does ANVISA review clean room validation documentation?
Yes. ANVISA requires proper clean room validation documentation during audits to ensure compliance with GMP and ISO standards.
How can Operon Strategist support my clean room project?
We provide complete solutions including design, validation, regulatory documentation, and ongoing consultation for medical device clean room projects in Brazil.
What standards govern medical device clean room design?
Medical device clean rooms must follow ISO 14644-1 for clean room classification and ISO 13485 for quality management systems, along with ANVISA requirements.