FDA 21 CFR Part 820 Quality System Regulation Consultant in Brazil
Overview of FDA 21 CFR Part 820 Quality System Regulation
The FDA 21 CFR Part 820 Quality System Regulation (QSR) outlines the requirements for medical device manufacturers to establish and maintain a Quality Management System (QMS) in compliance with U.S. FDA standards. These regulations ensure that medical devices are designed, manufactured, packaged, stored, and serviced with the highest quality and safety standards.
At Operon Strategist, we help Brazilian medical device manufacturers align with FDA QSR 21 CFR Part 820 requirements, enabling successful market entry into the United States.
What is FDA 21 CFR Part 820 Quality System Regulation?
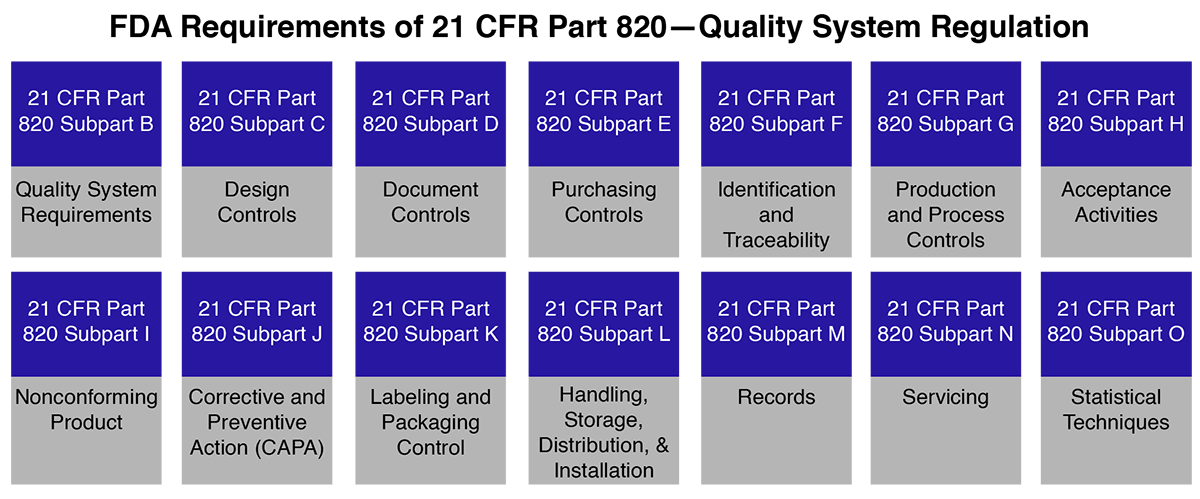
FDA 21 CFR Part 820 is a section of the U.S. Code of Federal Regulations specifically focused on quality system requirements for medical devices. It governs:
- Design and development
- Manufacturing and process controls
- Packaging and labeling
- Storage and distribution
- Installation and servicing of medical devices
Unlike ISO 13485 certification, FDA 21 CFR Part 820 does not have a certification process. Instead, manufacturers must demonstrate compliance through FDA inspections.

Why is FDA 21 CFR Part 820 Compliance Important?
For Brazilian medical device manufacturers targeting the U.S. market, compliance with 21 CFR Part 820 is mandatory. Non-compliance can lead to:
- FDA warning letters
- Import refusals
- Product recalls
- Severe damage to brand reputation
Adhering to FDA QSR ensures that your devices are safe, effective, and meet global regulatory expectations.
Why Should you Care About 21 CFR Part 820?
Medical device manufacturers must adhere to 21 CFR Part 820 regulations to avoid potential issues during inspections. Failing to meet these standards can result in an FDA warning letter, which can severely damage your reputation and adversely affect market performance. When importing devices from the U.S. to the U.S. market, it’s essential to ensure they comply with Quality System Regulation (QSR) requirements to avoid these risks.
How Operon Strategist Supports FDA 21 CFR Part 820 Compliance
We provide end-to-end consulting services to help manufacturers in Brazil achieve compliance:
✅ Gap Analysis – Assess your existing QMS against FDA 21 CFR Part 820 requirements
✅ QMS Implementation – Align processes with FDA standards
✅ Training Programs – Comprehensive training for your quality and regulatory teams
✅ Documentation Support – Guidance on preparing and maintaining FDA-compliant records
✅ Mock Audits – Simulated FDA inspections to identify and correct gaps
✅ Post-Inspection Support – Assistance in addressing FDA findings and ensuring continuous compliance
By working with us, manufacturers can establish robust quality systems and ensure smooth market entry into the United States.
Why Choose Operon Strategist?
- Expertise in FDA 21 CFR Part 820, FDA 510(k), and combination product regulations
- Experience across global regulatory authorities including ANVISA, US FDA, SFDA, EDA, and MHRA
- Tailored solutions for startups, SMEs, and large-scale manufacturers
- Proven success in helping medical device companies achieve U.S. market approvals
We are not just consultants—we are your regulatory partners in ensuring compliance, quality, and successful commercialization of medical devices.
We also provide medical device consultation for India, Saudi Arabia, the USA, the UK, South Africa, Oman, Iran & Egypt. Feel free to contact us.
FAQ
What is FDA 21 CFR Part 820?
FDA 21 CFR Part 820, also known as the Quality System Regulation (QSR), establishes the requirements for medical device manufacturers to ensure their products meet safety and efficacy standards before entering the U.S. market.
Who needs to comply with FDA 21 CFR Part 820?
All medical device manufacturers, including those in Brazil exporting to the U.S., must comply with FDA 21 CFR Part 820 to meet regulatory requirements and avoid enforcement actions such as warning letters or recalls.
How does Operon Strategist help with FDA 21 CFR Part 820 compliance?
Operon Strategist assists medical device manufacturers with gap analysis, QMS documentation, internal audits, risk management, and preparation for FDA inspections to ensure full compliance with 21 CFR Part 820.
What are the key elements of FDA 21 CFR Part 820?
Key elements of FDA 21 CFR Part 820 include design controls, production and process controls, CAPA (Corrective and Preventive Actions), supplier management, complaint handling, and internal audits.
What is the difference between ISO 13485 and FDA 21 CFR Part 820?
ISO 13485 is an international QMS standard for medical devices, while FDA 21 CFR Part 820 is a U.S. regulatory requirement. Compliance with ISO 13485 helps with FDA QSR compliance, but additional steps may be needed to meet FDA expectations.