Medical Device Registration in Costa Rica
What’s Stopping Medical Devices from Entering Costa Rica’s Fast-Growing Market?
Costa Rica has quickly established itself as a key destination for medical device manufacturing and export in Latin America. With government incentives, a highly skilled workforce, and modern infrastructure, the country offers attractive opportunities for medical technology companies worldwide.
However, successfully placing medical devices in this promising market depends on meeting local regulatory requirements. Medical device registration with the Costa Rican Ministry of Health is mandatory before products can be marketed or distributed. The process involves device classification, regulatory submissions, appointing a local representative, and ensuring technical and clinical documentation is in order — particularly for higher-risk devices.
Without the right strategy and expertise, navigating these regulatory procedures can delay market access and increase operational risks. Reliable regulatory support ensures seamless compliance, efficient registration, and faster entry into Costa Rica’s growing healthcare sector.
Why Is Medical Device Registration Important in Costa Rica?
Without completing the formal registration process, manufacturers cannot legally market, distribute, or sell medical devices within Costa Rica. Registration ensures product safety, efficacy, and compliance with national and international standards.Additionally, achieving regulatory approval opens up export opportunities through Costa Rica’s numerous trade agreements with the US, EU, and Latin American countries.

Medical Device Registration Process in Costa Rica
To legally market medical devices in Costa Rica, companies must complete the following steps:
- Device Classification
Products are classified based on risk level, following a system similar to Health Canada’s framework. - Local Representation
An authorized in-country representative is required to manage the registration process and ongoing regulatory responsibilities. - Regulatory Submission & Approval
Applications must include technical files, clinical data (if applicable), and proof of compliance with recognized international standards. - Expedited Registration for FDA-Listed Devices
Devices approved or listed with the US FDA may qualify for a simplified registration route, reducing approval timelines.
Classification of Devices in Costa Rica
Costa Rica’s medical device classification system is similar to Health Canada’s device classification system. The diagram below explains the classification system properly.
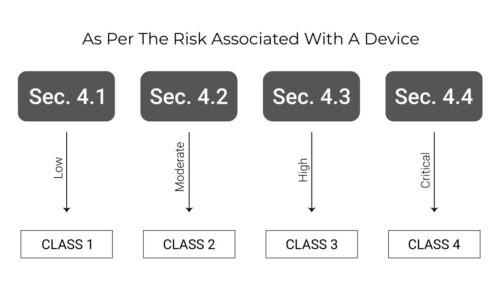
The registration process for Class 1 and Class 2 devices is simple, whereas Class 3 and Class 4 devices need higher requirements such as clinical studies performed for these devices. The class 2 and class 4 devices which are listed, cleared or approved with USFDA are also eligible for a simplified registration process.
How Operon Strategist Supports Market Access in Costa Rica?
Operon Strategist delivers tailored regulatory consulting services to support medical device registration and market placement in Costa Rica. The team manages the entire regulatory journey, from classification strategy to post-market compliance.
Services include:
- Device Classification Guidance
- Regulatory Strategy and Submission Management
- Quality Management System (QMS) Setup
- Technical Documentation Preparation
- In-Country Representation Coordination
- Post-Market Compliance Support
- Turnkey Project Consultation for Facility Setup
This comprehensive, hands-on approach ensures devices meet all Ministry of Health regulations while accelerating market entry.
Find the right service for your medical device project
Why Work with Operon Strategist?
Decades of combined experience in global medical device regulations make Operon Strategist a trusted partner for companies targeting Latin American markets. With a deep understanding of both international and Costa Rican regulatory frameworks, the team offers efficient, compliant, and market-driven solutions.
Key advantages include:
- Proven experience in medical device registration across multiple jurisdictions
- In-depth knowledge of Costa Rican Ministry of Health requirements
- Streamlined, end-to-end service delivery
- Custom solutions aligned with individual product portfolios and market strategies