What Is MDR Compliance?
MDR compliance refers to a manufacturer’s ability to meet the legal, technical, and clinical requirements outlined in EU MDR 2017/745 for medical devices intended for the European market. It covers product safety, performance, technical documentation, post-market surveillance, and vigilance systems.
In Germany, the Federal Institute for Drugs and Medical Devices (BfArM) and regional authorities closely monitor EU MDR adherence, ensuring patient safety and product traceability in the healthcare system.
Let's Grow Your Business Together
Why EU MDR Compliance Is Critical in Germany?
Germany holds a dominant position in the EU’s medical device sector. The nation’s hospitals, clinics, and distributors require all imported and locally manufactured devices to meet EU MDR 2017/745. Non-compliance risks include market withdrawal, financial penalties, legal action, and loss of CE Mark certification.
To mitigate these risks, manufacturers must stay current with regulatory updates and implement a structured MDR Compliance checklist.
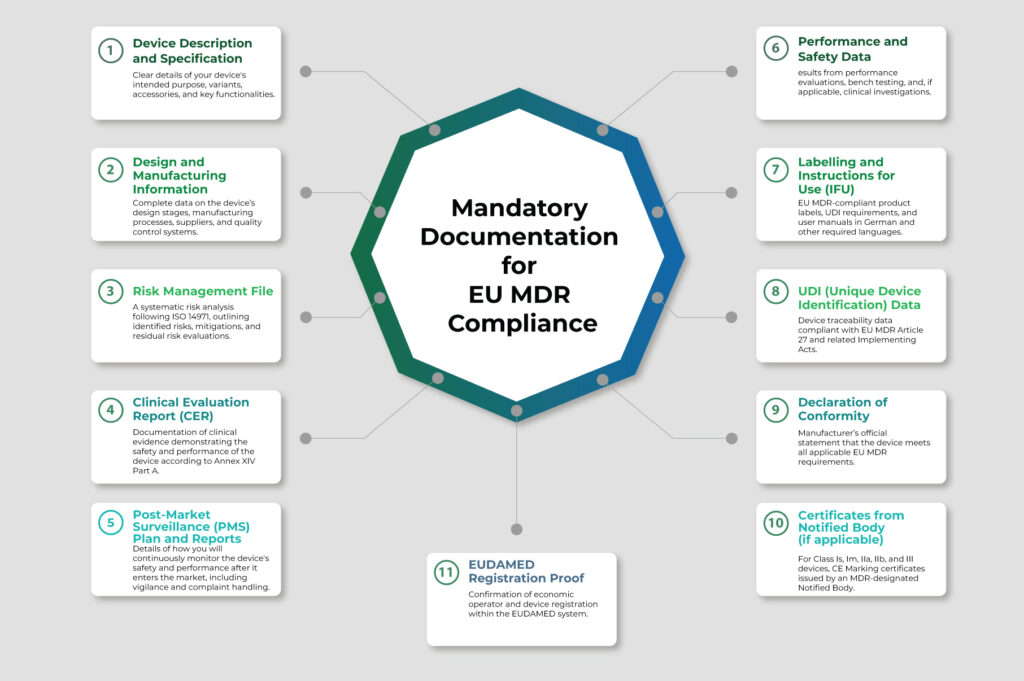
EU MDR Compliance Checklist for Germany: 2025 Edition
- Classify Your Medical Device Correctly
Identify your device’s risk classification under EU MDR: Class I, IIa, IIb, or III. Germany strictly follows the EU MDR classification rules (Annex VIII). Misclassification can cause delays and non-approvals. - Appoint an Authorized Representative in Germany/EU
Non-EU manufacturers must appoint a European Authorized Representative (EC REP) to manage regulatory communications and device registration in the EU, including Germany. - Conduct Clinical Evaluation and Investigations
Prepare clinical evidence as per Annex XIV Part A of EU MDR. High-risk devices need clinical investigations and ongoing Post-Market Clinical Follow-up (PMCF). - Establish a Post-Market Surveillance (PMS) System
Create a proactive PMS plan aligned with Articles 83-86 of EU MDR. It should include vigilance reporting, complaint handling, and Periodic Safety Update Reports (PSUR) for higher-class devices. - Obtain Notified Body CE Certification (If Applicable)
For Class Is, Im, IIa, IIb, and III devices, certification from an EU Notified Body is mandatory. - Register Devices and Economic Operators on EUDAMED
Use EUDAMED for mandatory registration of devices, manufacturers, authorized representatives, and importers. - Update Labelling and UDI Requirements
Ensure product labelling and packaging comply with Annex I Section 23 of EU MDR, incorporating the Unique Device Identification (UDI) system. - Prepare for Regular EU MDR Audits in Germany
German authorities and Notified Bodies will inspect your compliance. Keep documentation and records audit-ready at all times. - Stay Updated on Germany-Specific EU MDR Amendments
Monitor BfArM and national legislation for updates or local implementations of EU MDR.
Partner with Operon Strategist for smooth EU MDR Compliance in Germany!
How Operon Strategist Supports Your EU MDR Compliance in Germany?
At Operon Strategist, we offer complete consulting services for medical device manufacturers aiming for smooth, error-free EU MDR Compliance in Germany. Our expertise covers:
With an experienced team and proven track record, Operon Strategist ensures your business stays aligned with the latest EU MDR requirements and German regulatory expectations.
FAQ'S
The checklist includes device classification, technical documentation (Annex I–III), UDI & EUDAMED registration, clinical evaluation, post-market surveillance, and appointing a PRRC.
Registration in the European database (EUDAMED) enables traceability of devices, regulatory oversight, and distribution control across the EU.
Manufacturers must perform a clinical evaluation report (CER) based on clinical data and comply with MDR risk and performance requirements.
Yes. Devices previously certified under MDD/AIMDD must be re-assessed for EU MDR compliance and meet new requirements.
Non‑compliance can lead to delay or rejection by Notified Bodies, suspension of CE marking, and restricted market access.