Clean Room Design Consultant for Medical Devices in Germany – Operon Strategist
Why Clean Room Design Matters for Medical Devices?
Clean Room Design is a critical factor for medical device manufacturers in Germany who must comply with global quality and safety regulations. A properly designed clean room ensures controlled environmental conditions—such as air quality, humidity, and temperature—that prevent contamination and safeguard product integrity.
At Operon Strategist, we specialize in regulatory-compliant clean room design tailored to the unique requirements of medical device manufacturing. Our solutions enable manufacturers to meet ISO 14644-1 standards, maintain ISO 13485 quality systems, and achieve EU MDR compliance.
Let's Grow Your Business Together
What is a Clean Room?
A clean room is a specialized enclosed facility engineered to minimize contaminants such as dust, microbes, chemical vapors, and aerosol particles. These environments are essential for industries where precision and sterility are non-negotiable, including:
- Medical devices
- Pharmaceuticals
- Biotechnology
- Diagnostics & R&D labs

By maintaining strict controls over airflow, temperature, and entry protocols, clean rooms protect product safety and ensure compliance with international regulations.
Clean Room Regulations for Medical Devices in Germany
For medical device manufacturers, compliance with clean room regulations is mandatory. Key frameworks include:
- ISO 14644-1 – Specifies clean room classification by particle count.
- ISO 13485 – Requires controlled environments for safe medical device production.
- EU MDR – Enforces regulatory standards for patient safety and device reliability.
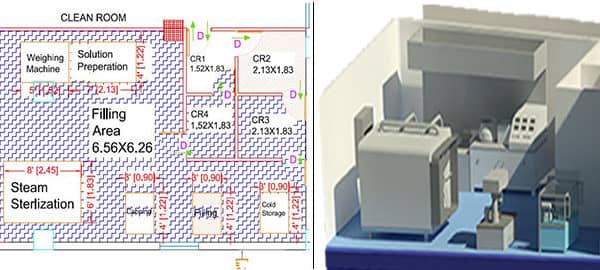
At Operon Strategist, we help manufacturers design clean rooms that meet these global benchmarks, ensuring both compliance and operational efficiency.
How Operon Strategist Supports Clean Room Design in Germany
Our clean room consulting services combine technical expertise with regulatory knowledge to deliver end-to-end solutions:
- AutoCAD Clean Room Design – Custom layouts for medical device plants, packaging areas, and laboratories.
- Regulatory Consulting – Guidance on ISO 14644-1, ISO 13485, EU MDR, and other applicable standards.
- Clean Room Validation – Full validation support to prove compliance and operational readiness.
Key Features of Our Clean Room Design Services
Our approach ensures your facility is compliant, efficient, and cost-effective. We focus on:
- Air Quality Control – Designing ventilation systems to maintain minimal particle levels.
- Environmental Management – Optimizing temperature, humidity, and differential pressure.
- Controlled Entry Systems – Implementing gowning/de-gowning procedures.
- Process Efficiency – Delivering both short- and long-term clean room improvement strategies.
Why Choose Operon Strategist for Clean Room Design in Germany?
- Global Expertise with Local Focus – Decades of experience in clean room design for regulated industries.
- ISO 14644-1 & ISO 13485 Compliance – Helping manufacturers align with international standards.
- Proven Track Record – Successful clean room projects across Europe, the Middle East, and Asia.
Partnering with Operon Strategist ensures your clean room is designed to meet both regulatory requirements and production efficiency goals.
Partner with Operon Strategist for Your Next Clean Room Project
From planning and design to validation and compliance, Operon Strategist is your trusted clean room design consultant in Germany. We help manufacturers achieve audit-ready facilities that ensure quality, safety, and market success.
📩 Contact us today at enquiry@operonstrategist.com or reach us via WhatsApp to discuss your clean room project.
Let's Grow Your Business Together
Ensure Compliance with Expert Clean Room Design.
FAQ'S
Clean room guidance ensures that medical device manufacturers follow international standards like ISO 14644 for design, validation, and maintenance of controlled environments to prevent contamination.
Clean room consulting helps companies design compliant layouts, maintain regulatory standards, and prepare for inspections under FDA, EU MDR, and ISO guidelines.
Operon Strategist provides clean room design consulting, ISO 14644 classification, HVAC and layout guidance, validation documentation, and regulatory compliance support.
Key regulations include ISO 14644, US FED STD 209E, EU MDR requirements, and FDA clean room standards.
We prepare the validation plan, support IQ/OQ/PQ documentation, and ensure your clean room setup meets all regulatory requirements for audits and inspections.