FDA 21 CFR Part 820 Compliance Consulting for Medical Devices
21 CFR Part 820 Quality System Regulation (QSR)
FDA 21 CFR Part 820 is a key regulation that defines the Quality System Regulation (QSR) for medical device manufacturers. It establishes current Good Manufacturing Practice (cGMP) requirements to ensure that devices are designed, manufactured, packaged, labeled, stored, installed, and serviced in a way that guarantees safety, effectiveness, and compliance.
At Operon Strategist, we provide specialized consulting services to help medical device manufacturers achieve 21 CFR Part 820 compliance with confidence. Our solutions align your Quality Management System (QMS) with FDA expectations so your devices meet the highest standards of quality and regulatory approval.
What is FDA 21 CFR Part 820 Quality System Regulation?
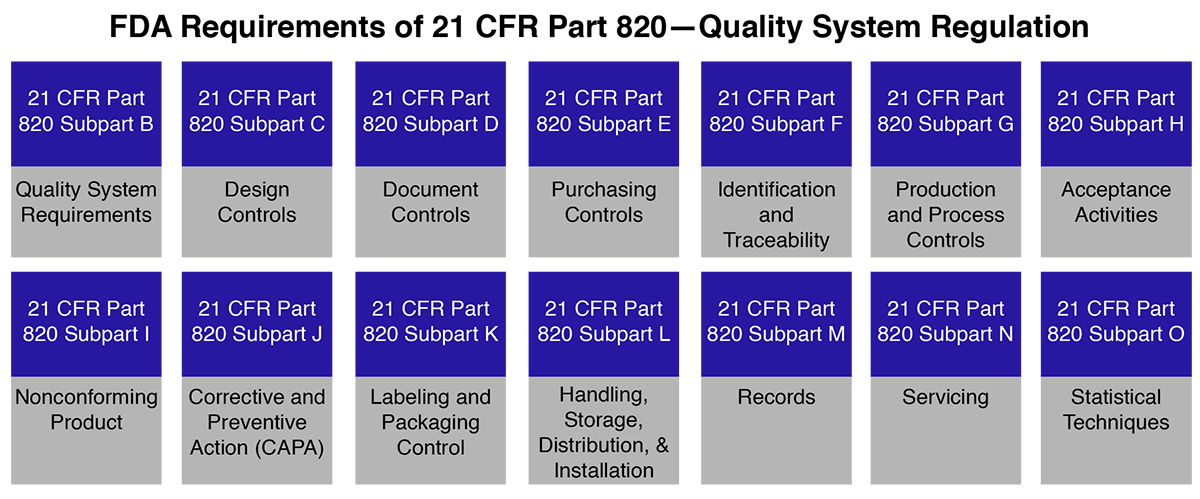
he Code of Federal Regulations (CFR) Title 21 governs medical devices in the U.S., with Part 820 specifically covering QSR requirements. Unlike other certifications, there is no direct “21 CFR Part 820 certification.” Instead, manufacturers must demonstrate compliance during FDA inspections.
This regulation covers every aspect of medical device operations, including:
Design and development
Manufacturing and process controls
Packaging and labeling
Storage and distribution
Installation and servicing
By complying with 21 CFR Part 820, manufacturers can avoid costly compliance issues, FDA warning letters, or import restrictions that can disrupt business operations.
Let's Grow Your Business Together

Why Compliance with 21 CFR Part 820 Matters?
Non-compliance with FDA 21 CFR Part 820 can lead to:
FDA warning letters and enforcement actions
Delays in product launch or market entry
Damaged brand reputation and customer trust
Business losses due to import bans or recalls
Whether you are manufacturing in the U.S. or exporting devices to the U.S. market, compliance with 21 CFR Part 820 QSR is not optional—it’s mandatory for ensuring smooth regulatory approval and sustainable market performance.
To Discuss Your Compliance Needs And Secure Your Pathway To FDA Approval.
Our Expertise in 21 CFR Part 820 Compliance
Operon Strategist helps medical device manufacturers build, strengthen, and maintain compliance with 21 CFR Part 820 through end-to-end consulting services.
Our support includes:
Gap Analysis – Assessing your existing QMS against 21 CFR Part 820 requirements
Documentation Support – Preparing and implementing compliant procedures, records, and technical files
Training Programs – Educating teams on FDA QSR requirements for smooth adoption
Mock Audits & Inspections – Identifying compliance gaps before FDA inspections
Post-Inspection Support – Addressing non-conformities and ensuring corrective actions
With our expertise, you can establish a robust QMS that meets FDA expectations, avoids compliance risks, and strengthens your competitive position in the U.S. medical device market.
Why Choose Operon Strategist?
Proven Experience: Decades of consulting for global medical device manufacturers.
Comprehensive Regulatory Knowledge: Expertise in FDA 21 CFR Part 820, 510(k) submissions, drug-device combination products, and GMP regulations (21 CFR 210, 211, and 4).
End-to-End Guidance: From QMS setup to FDA inspection readiness.
Client-Centric Approach: Customized solutions tailored to your device category and business needs.
We are also trusted FDA 510(k) clearance consultants, supporting clients with dossier preparation, query resolution, and registration of Small Business Units (SBUs).
Partner with Operon Strategist for FDA 21 CFR Part 820 Compliance
Ensuring compliance with 21 CFR Part 820 is critical to your U.S. market success. Operon Strategist empowers you to confidently navigate regulatory challenges, build strong quality systems, and achieve faster market entry.
Take The First Step Toward FDA 21 CFR Part 820 Compliance
FAQ'S
FDA 21 CFR Part 820, also known as the Quality System Regulation (QSR), establishes current Good Manufacturing Practice (cGMP) requirements for medical device manufacturers to ensure product safety, quality, and regulatory compliance.
No, there is no certification for 21 CFR Part 820. Instead, manufacturers must demonstrate compliance during FDA inspections to ensure their quality systems meet regulatory requirements.
Compliance with 21 CFR Part 820 is mandatory for medical device manufacturers selling in the U.S. market. Non-compliance can result in FDA warning letters, import restrictions, product recalls, or delays in market entry.
Operon Strategist assists medical device manufacturers with gap analysis, documentation, training, mock audits, and post-inspection support to ensure full compliance with FDA 21 CFR Part 820 requirements.
Yes, 21 CFR Part 820 applies to all finished medical devices intended for human use that are marketed in the United States, covering design, manufacturing, packaging, labeling, storage, installation, and servicing.