FDA 21 CFR Part 820 Quality System Regulation
Operon Strategist provides complete support for 21 CFR Part 820 compliance, helping Algerian medical-device manufacturers align their Quality Management System with U.S. FDA requirements. Our team assists in documentation, training, gap analysis, and FDA QSR audit readiness to ensure your facility meets all FDA QSRs for design, manufacturing, and process control. With our expert consulting, you can confidently prepare for FDA inspections, maintain product quality, and achieve seamless access to the U.S. medical-device market.
What is FDA 21 CFR Part 820 Quality System Regulation?
FDA 21 CFR Part 820 is the Quality System Regulation (QSR) that outlines cGMP requirements for medical devices in the U.S. It covers all aspects of design, manufacturing, packaging, labeling, and servicing. While there’s no certification, FDA inspects manufacturers for compliance essential for market access in the U.S. Algerian manufacturers aiming to export to the U.S. must implement a robust QMS aligned with these requirements. Operon Strategist supports companies in achieving full compliance through expert guidance and tailored solutions.

FDA QSR Compliance for Medical Device Manufacturers:
FDA 21 CFR Part 820 is a key component of the Current Good Manufacturing Practices (cGMP) regulations for medical devices.
Authorized under Section 520(f) of the Federal Food, Drug, and Cosmetic Act, this regulation commonly known as the Quality System Regulation (QSR) specifies the quality system requirements that medical device manufacturers must follow to ensure product safety and effectiveness.
The QSR governs the methods, facilities, and controls used in the design, manufacturing, packaging, labeling, storage, installation, and servicing of medical devices intended for human use in the U.S. market.
Compliance with FDA 21 CFR Part 820 is mandatory and verified through FDA inspections, making it essential for manufacturers aiming to export to the U.S. Operon Strategist assists manufacturers in aligning with these requirements to ensure readiness for FDA audits and successful market entry.
Why Should you Care About 21 CFR Part 820?
For Algerian medical device manufacturers targeting the U.S. market, compliance with FDA 21 CFR Part 820 is critical. Failure to meet these Quality System Regulation (QSR) requirements during an FDA inspection can result in warning letters, product holds, and damage to your company’s reputation and market prospects.
Additionally, for Algerian importers and distributors handling U.S.-origin medical devices for markets like Saudi Arabia, verifying compliance with FDA QSR standards is essential. It not only fulfills regulatory obligations but also ensures the safety and quality of devices entering regional markets. Partnering with experts like Operon Strategist can help you navigate these compliance challenges effectively.
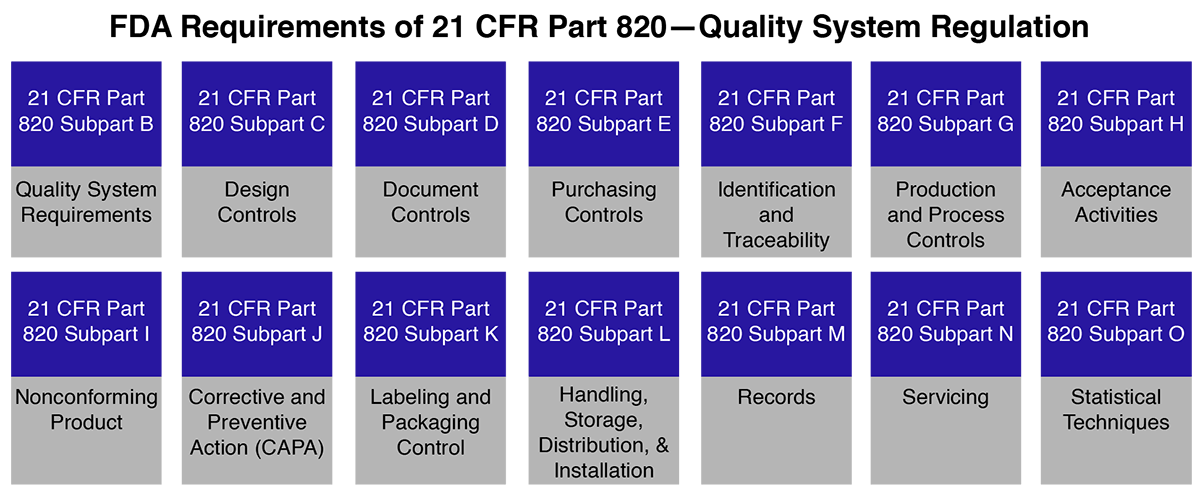
Our Role in FDA 21 CFR Part 820 – Quality System Regulation Compliance
Planning to export your medical devices to the United States from Algeria? Compliance with FDA’s 21 CFR Part 820 Quality System Regulation (QSR) is mandatory even if you already have an existing QMS. Your system must align specifically with FDA requirements to gain market access.
Operon Strategist supports Algerian manufacturers throughout the entire compliance journey. We begin with a gap analysis to assess your current system, provide in-depth training on 21 CFR Part 820, assist in preparing and implementing required documentation, and conduct mock audits to ensure readiness. We also offer post-inspection support to resolve any FDA-identified non-conformities.
With our expert guidance, you can build a robust, FDA-compliant quality system that ensures product quality, regulatory approval, and successful entry into the U.S. market.
Why Choose Operon Strategist?
Operon Strategist is a trusted FDA 510(k) clearance consultant, helping medical device manufacturers especially Small Business Units (SBUs) navigate the complex regulatory landscape for U.S. market entry. From determining product testing requirements and preparing the 510(k) dossier to resolving FDA queries, we guide clients through every step until successful approval is obtained.
As a specialized medical device consulting firm, we also support the registration of drug-device combination products. Our expertise spans regulatory requirements for drugs (21 CFR Parts 210 & 211), devices (21 CFR Part 820), and combination products (21 CFR Part 4). What sets us apart is our in-depth knowledge, proven methodology, and global experience.
We offer regulatory consulting services not only in Algeria but also in India, Saudi Arabia, the USA, the UK, South Africa, Oman, Iran, and Egypt. Get in touch with us to ensure smooth, compliant, and successful product registrations.
Ready to Get FDA 510(k) Clearance?
FAQ
What is FDA 21 CFR Part 820?
It is a U.S. regulation that defines quality system requirements for medical devices. Compliance ensures products are safe, effective, and eligible for the U.S. market.
Is certification required?
There is no formal certification for 21 CFR Part 820. However, FDA inspects manufacturers to verify compliance.
How does Operon Strategist help?
We assist Algerian manufacturers with gap analysis, QMS implementation, documentation, training, and audit readiness. Our support ensures smooth U.S. market entry.
Is ISO 13485 enough for U.S. market?
No, ISO 13485 is not a substitute for FDA QSR compliance. Specific FDA requirements must still be addressed.
Do you support FDA 510(k) submissions?
Yes, we provide end-to-end FDA 510(k) consulting including dossier preparation and SBU registration. We also help resolve FDA queries.
