Introduction: Why Medical Device Quality Assurance Matters in Algeria
As Algeria continues to strengthen its healthcare infrastructure and local manufacturing capabilities, the need for Medical Device Quality Assurance (QA) is more vital than ever. Whether it’s a diagnostic device or a complex surgical tool, the quality, safety, and performance of medical devices directly affect patient health outcomes.
For Algerian medical device manufacturers aiming to enter regulated markets like the EU, GCC, or the USA, a robust Quality Assurance system is non-negotiable. It ensures compliance with international regulations such as ISO 13485:2016, EU MDR 2017/745, and FDA 21 CFR Part 820.
Looking For a Medical Device Regulatory Consultant?
What is Medical Device Quality Assurance?
Medical Device Quality Assurance refers to the system of procedures and protocols that ensure medical devices are designed, developed, manufactured, and distributed in compliance with applicable standards and regulations. It covers every phase—from raw material sourcing and design to post-market monitoring.
Key Components of a Medical Device QA System
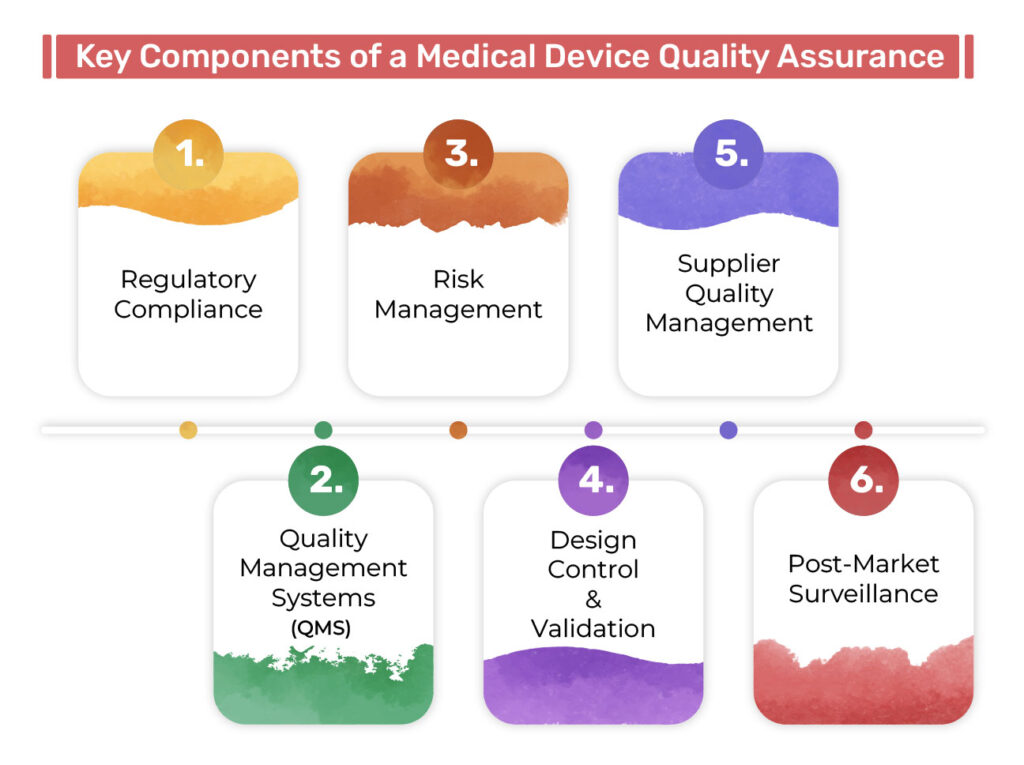
✅ Regulatory Compliance
Algerian manufacturers looking to export medical devices must comply with global standards like:
- ISO 13485:2016 – Quality Management System for medical devices
- EU MDR 2017/745 – European Union regulations
- FDA 21 CFR Part 820 – US FDA Quality System Regulation
- ISO 14971 – Risk Management in medical devices
These frameworks ensure that the devices are safe, effective, and reliable.
✅ Quality Management System (QMS)
A QMS is the backbone of quality assurance. It defines controlled processes such as:
- SOPs (Standard Operating Procedures)
- Design Controls
- Document Control
- CAPA (Corrective and Preventive Actions)
- Internal Audits
- Training Management
✅ Risk Management
Using ISO 14971, risk analysis begins early in the product design phase and continues throughout the lifecycle. This minimizes patient harm and supports regulatory approvals.
✅ Design Control & Validation
Manufacturers must document all phases:
- Design Inputs & Outputs
- Verification & Validation
- User Needs & Intended Use
This ensures consistent product performance and safety.
✅ Supplier Quality Management
QA isn’t limited to in-house operations. Vendor and supplier qualification, audits, and ongoing monitoring are crucial to prevent quality lapses due to non-compliant components.
✅ Post-Market Surveillance
Monitoring device performance after release is vital to ensure long-term safety. This includes tracking complaints, adverse events, and implementing corrective actions promptly.
Importance of QA for Algerian Medical Device Manufacturers
Implementing a robust QA system offers several advantages for Algerian companies:
- Faster Regulatory Approvals – Meet requirements for CE Marking, FDA 510(k), and SFDA registration.
- Product Safety & Patient Trust – Minimize risks of malfunction and enhance credibility.
- Global Market Access – Easily penetrate EU, USA, and Middle Eastern markets.
- Improved Business Reputation – Reduce recalls, penalties, and gain stakeholder trust.
- Process Efficiency – Well-structured processes lead to cost savings and fewer delays.
Ready to Build a Strong Quality Assurance System in Algeria?
Operon Strategist: Your QA System Consultant in Algeria
At Operon Strategist, we help Algerian medical device manufacturers establish and improve their Medical Device Quality Assurance systems. Our team ensures your processes align with both local and international regulatory standards.
Our QA Services Include:
- Complete QMS development as per ISO 13485:2016
- Gap analysis and internal audits
- Supplier qualification & compliance audits
- Support for FDA 510(k), CE Marking, and SFDA registration
- Assistance in design control, validation, risk management, and CAPA
- Documentation preparation for CDSCO (India) and other global authorities
