Medical Device Design and Development Services in Algeria
What Is Medical Device Design and Development?
Medical device design and development is a structured, innovation-driven process that transforms clinical needs into safe, effective, and compliant healthcare solutions. It involves taking a concept from initial idea through research, design, prototyping, testing, regulatory approvals, and manufacturing.
The process ensures that devices:
Meet user needs and clinical requirements
Comply with global regulations such as ISO 13485:2016 and FDA 21 CFR Part 820.30
Provide reliable, safe, and market-ready solutions
In essence, it is not just about creating a device—it’s about engineering solutions that improve patient outcomes, minimize risks, and earn regulatory and market trust.
Let's Grow Your Business Together
Key Pillars of Medical Device Design and Development
- Market Demand: Identify unmet medical needs and align device features with current clinical practices and healthcare trends.
- Clinical Impact: Define the device’s purpose and how it enhances diagnosis, treatment, or patient care.
- User & Patient Safety: Integrate risk management, usability engineering, and validation protocols to ensure safety throughout the product lifecycle.
From concept to prototyping and commercial production, successful medical device development balances innovation, user needs, and regulatory compliance, ensuring devices not only perform but also earn market trust.

Importance of Medical Device Design and Development
Medical device design is essential for advancing healthcare by:
Improving patient care and addressing unmet medical needs
Enhancing usability to reduce errors and simplify operation
Ensuring compliance with global standards like ISO 13485:2016 Clause 7.3 and FDA 21 CFR Part 820.30
Driving innovation and enabling next-generation medical solutions
Thoughtful design leads to safer, more effective diagnostics and treatments, catering to diverse healthcare challenges.
The Medical Device Design And Development Process Guide
During the design and development stage of the medical device design process, we assist various medical device manufacturing industries in Algeria to ensure that appropriate steps are taken to meet the regulatory compliance of the medical device design and development. After analyzing a new medical device, the next step in its product development is the medical device design. This is the most important stage in medical device development for a flawed design may ahead of it being ineffective or risky. At the medical device design stage, a design control process system is required.
Being, design controls are simple and logical steps to ensure that what you develop is what you determine to develop and that the final product meets your customer’s needs and expectations.
- Medical device development uses a ton of comparative parts in a large number of various medical devices. A solid definition extricated by dissecting the market needs. When you’re finished with the products definition and thought, you have to consider systems like FDA has characterized and licensed innovation rights. Medical devices classification depends on the hazard related to the utilization and upheld by law. So as to get into the market, the medical devices need to go through certain administrative compliances, subject to both provincial and worldwide guidelines. Medical devices measures are useful and upheld by law in indicating and assessing the prerequisite for structure and execution parameters for biomedical materials, apparatuses, and gear. These medical devices standards permit establishments in the medical device field, for example, products manufacturers, research centres, and others to review and survey such hardware and devices to guarantee standard quality and ease of use.
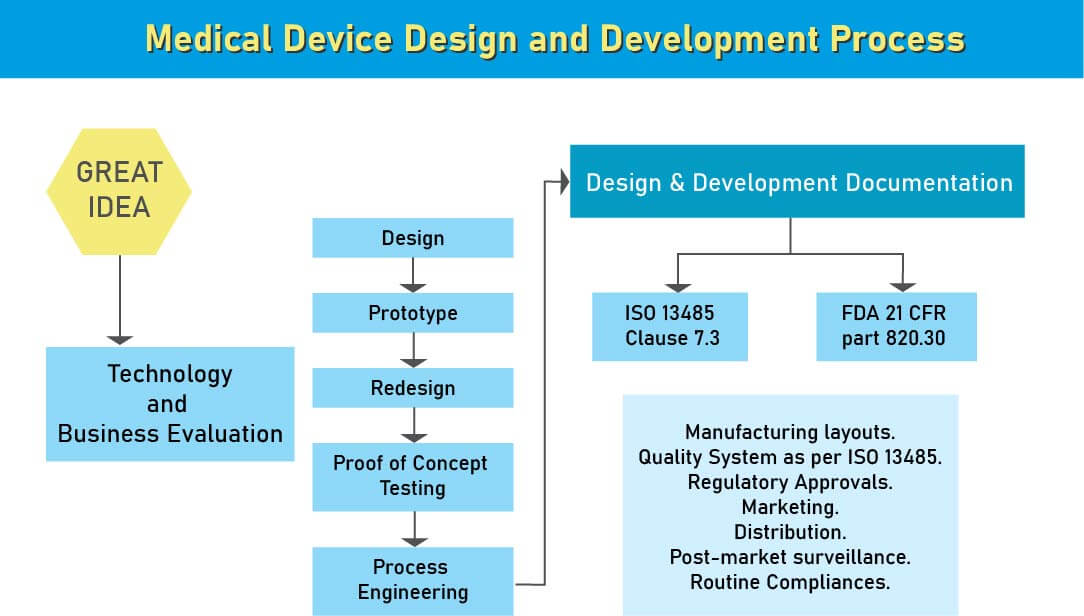
- The International Organization for Standardization likewise have details for medical devices principles. ISO 13485 is broadly utilized guidelines over the world for medical devices quality administration. Other than these worldwide models, there are sure gauges which are area explicit and every one of them are embraced from universal norms with little adjustment and constraint.
- Medical devices manufacturers need to pursue Design Control rules since the administrative bodies like FDA, European Commission, and others need to guarantee that the medical devices are alright for potential clients before makers begin to advertise the devices. Beginning stage from which Design Control starts is Design Input advancement and endorsement, which comprises of device design and manufacturing procedures to be completed in the generation stage. Design control is a comprehensive methodology and doesn’t end with moving the design to the generation stage when the plan is settled. It additionally affects manufacturing procedures as indicated by the adjustments in the design stage or even after creation input. It is a progressing procedure to build up a product that is usable for a client and in this way for the improved product, it considers progressive changes from utilization design just as breaking down failed items.
Medical Device Design Services & Product Development Process?
- Feasibility
- Planning
- Design and development
- Verification
- Validation
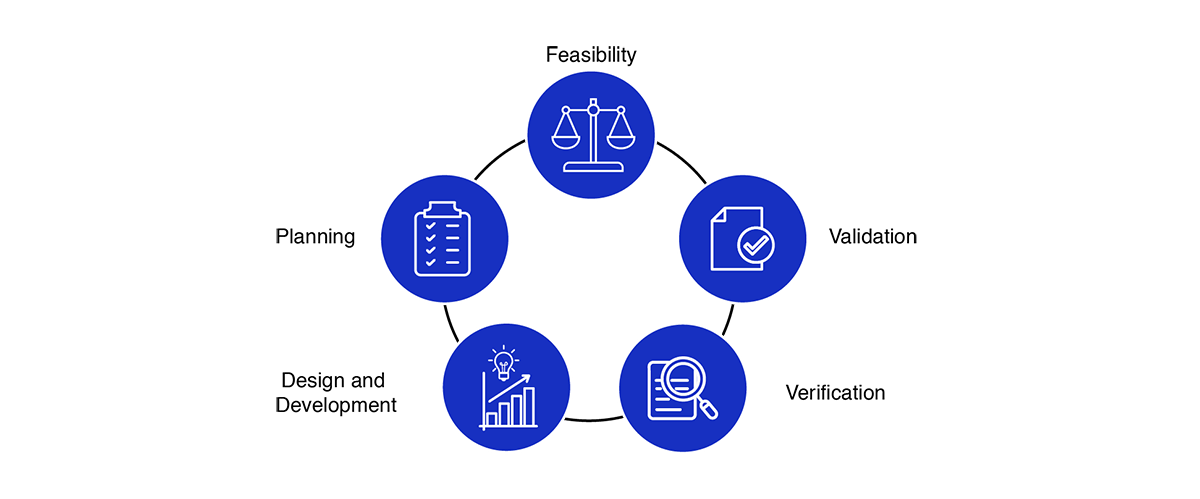
Medical device design services and development process encourages an early focus on clear problem definition and de-risking a wide variety of potential solutions. By later phases, the funnel of medical device design options narrows significantly, converging on a final product that has been thoroughly shown to meet the customer needs and is ready for distribution.
Medical Device Design Control
Design control is a structured approach mandated by the U.S. FDA for Class II and III devices. It involves documented processes that demonstrate:
- Safety and effectiveness
- Compliance with user needs and regulatory requirements
- Systematic verification and validation of designs
Design control ensures that what is developed matches the intended requirements, reducing risks of failure and regulatory non-compliance.
Medical Device Product Development in Algeria
Product development covers the complete lifecycle from concept to market. Key stages include:
- Opportunity identification and market analysis
- Concept generation and selection
- Design, prototyping, and engineering
- Regulatory compliance and approvals
- Manufacturing and commercialization
- Post-market surveillance
Each phase is critical to ensure the device is safe, effective, and aligned with user needs and international standards.
The Medical Device Design and Development Services Includes :
Combination Product - Drug - Device
Each manufacturer of Drug Device combination products (e.g. Drug, device combination products like prefilled syringes, applicators of the tropical products) shall have adequate design and development activity done so as to prove the adequacy of the safety and efficacy of the product. The medical device design and development activity is the systematic methodology, which establishes the proper medical device design and development.
Medical Device Design Control
After conceptualizing a new medical device, the next step in its product advancement is the design. This is the most important stage in the advancement of a medical device since a defective plan may prompt it being inadequate or dangerous (that is, not affirmed or cleared by the administrative organization). At the medical device design stage, an outline control process should be started and actualized as a feature of the quality system requirement.
Accelerate Your Medical Device Design and Development in Algeria – Contact Operon Strategist Today!
Our Role in Medical Device Design Control in Algeria
Operon Strategist supports medical device manufacturers in Algeria throughout the design and development lifecycle, ensuring products are safe, effective, and globally compliant. Our services align with ISO 13485:2016, FDA 21 CFR Part 820.30, EU MDR, and UKCA requirements, helping manufacturers achieve smooth regulatory approvals and faster market entry. From design controls, risk management, and verification & validation to combination product support, cleanroom-ready manufacturing design, and regulatory documentation, we provide end-to-end guidance that transforms innovative concepts into market-ready medical devices for Algeria, the EU, the UK, and other global markets.
FAQ'S
What is medical device design and development?
Medical device design and development is the process of creating medical products that are safe, effective, and meet user needs. It includes concept planning, risk management, prototyping, testing, regulatory compliance, and manufacturing.
Why is design control important in medical device development?
Design control ensures that medical devices are developed systematically and meet regulatory standards such as ISO 13485 and US FDA 21 CFR Part 820.30. It helps prevent errors, ensures patient safety, and facilitates smoother market approvals.
Are design controls required for medical device manufacturers in Algeria?
Yes, Algerian manufacturers aiming to export devices must implement design controls as per international regulatory expectations like CE marking (EU), UKCA (UK), and FDA (USA) to ensure compliance and market access.
How can Operon Strategist help with medical device design in Algeria?
Operon Strategist offers end-to-end consulting for medical device design and development. We assist with tool validation, QMS setup, risk management, and training to help Algerian manufacturers comply with ISO 13485 and other global standards.
What is the first step in developing a medical device in Algeria?
The first step is identifying the medical need and defining user requirements. From there, Operon Strategist helps you develop a regulatory strategy, classify your device, and begin the design control process.