SFDA Medical Device Registration Consultants for Algerian Manufacturers
Operon Strategist provides expert SFDA medical device registration consulting for Algerian manufacturers aiming to enter the Saudi Arabian market. Our services cover the complete Medical Device Marketing Authorization (MDMA) process, including device classification, preparation of SFDA-compliant technical dossiers, ISO 13485 QMS compliance, and appointment of a Saudi-based authorized representative. We assist with online MDMA registration, clinical data review, quality system audits, and post-market surveillance (PMS) obligations, ensuring a smooth and timely approval process. With in-depth knowledge of Saudi regulations, including the latest updates from the Saudi FDA, we help Algerian medical device companies achieve regulatory compliance, gain market access, and maintain ongoing vigilance reporting with confidence.
Why Partner with SFDA Medical Device Registration Consultants?
SFDA registration requires in-depth knowledge of regulations, procedures, and documentation. Our consultants provide:
- Expert knowledge of Saudi medical device regulations and recent updates.
- Compliance support for documentation, QMS, and ISO 13485 certification.
- End-to-end guidance through Medical Device Marketing Authorization (MDMA) approval.
- Efficient process handling, reducing regulatory complexities.
With professional guidance, Algerian manufacturers can enter the Saudi Arabian healthcare market confidently and compliantly.

SFDA Medical Device Registration – An Overview
The SFDA acts as the national regulatory authority for medical devices and IVDs. All devices require Medical Device Marketing Authorization (MDMA) approval before being placed in the Saudi market.
Between 2019 and 2022, Saudi Arabia updated its regulations, impacting device classification and MDMA requirements. As a result, previously approved devices must now comply with the revised framework.
Operon Strategist continuously monitors these regulatory updates to provide Algerian manufacturers with the most accurate compliance strategies.
Medical Device Regulations in Saudi Arabia
- Before entering the Saudi market, Algerian manufacturers must ensure compliance with the following:
- Regulatory Authority: Saudi Food & Drug Authority (SFDA)
- Regulation: Royal Decree No. (M/54)
- Regulatory Pathway: MDMA Approval
- Authorized Representative: Saudi-based Representative is mandatory
- QMS Requirement: ISO 13485:2016 certification
Looking for Consultant?
Let’s have word about your next project
Medical Device Marketing Authorization (MDMA)
The MDMA system manages device approvals. Most device classes require MDMA before entering the market.
- Timeline: Average of 35 days for approval.
- Validity: Registration remains valid for 3 years (or for the original specified duration).
SFDA Device Classification Rules
General Medical Devices
The SFDA follows the MDS-G5 guidelines, similar to the European MDR framework. Devices are classified into Class A, B, C, or D, based on risk.
| SFDA Class | Risk Level | MDR Equivalent | Example |
|---|---|---|---|
| A | Low | Class I | Low-risk general devices |
| A – S | Low-medium | Class Is | Sterile devices |
| A – M | Low-medium | Class Im | Measuring devices |
| A – R | Low-medium | Class Ir | Reusable surgical instruments |
| B | Low-medium | Class IIa | Active devices |
| C | Medium-high | Class IIb | More invasive devices |
| D | High | Class III | High-risk, life-supporting devices |
In Vitro Diagnostics (IVDs)
The SFDA also aligns with the European IVDR.
| SFDA Class | Risk Definition | Example |
|---|---|---|
| A | Low risk | Routine IVDs |
| B | Moderate risk | Infectious disease tests |
| C | High individual risk | Blood screening devices |
| D | High public health risk | HIV/HBV/HCV tests |
SFDA Medical Device Registration Process for Algerian Manufacturers
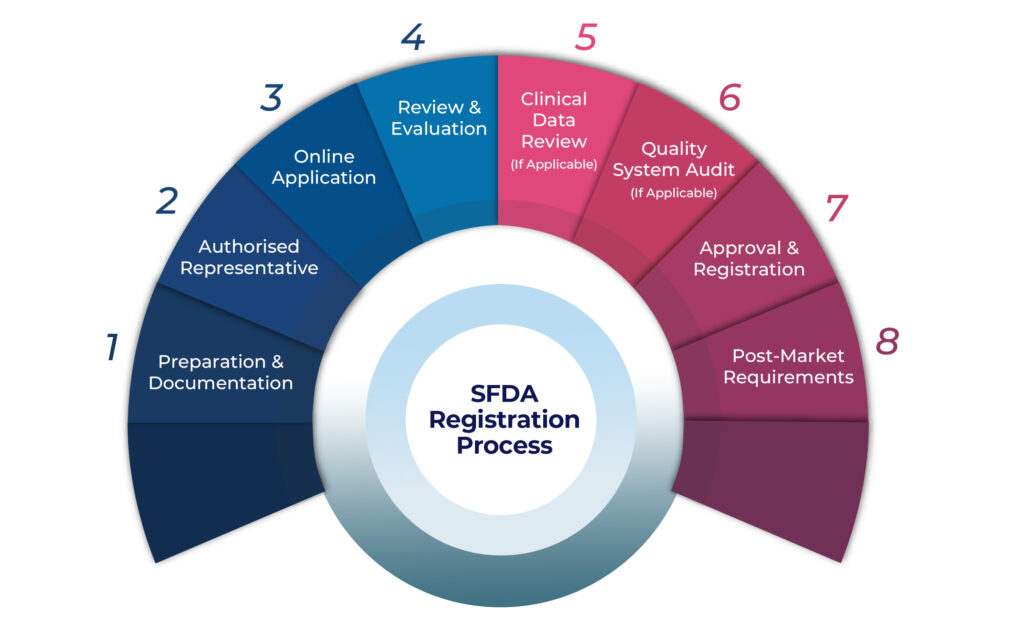
Preparation & Documentation
Identify device classification per SFDA rules
Prepare a complete technical file with design, labeling, and clinical data
Ensure ISO 13485 QMS compliance
Appoint an Authorized Representative
Foreign manufacturers must assign a local Saudi representative
Online Application
Submit application via the SFDA MDMA portal
Review & Evaluation
SFDA reviews technical, clinical, and quality documentation
Clinical Data Review (if required)
For new or high-risk devices, additional evidence may be requested
Quality System Audit (if required)
SFDA may conduct a QMS audit of your manufacturing facility
Approval & Registration
Upon successful review, SFDA issues the registration certificate
Post-Market Surveillance (PMS)
Ongoing obligations include vigilance reporting and adverse event tracking
Documentation Required for SFDA Registration
All documents must be submitted in English via the MDMA portal. Required documentation includes:
- Manufacturer details and Saudi Authorized Representative information
- Device description, intended use, labeling, and promotional materials
- Proof of approval/market authorization in a GHTF country
- Clinical data (if applicable) to prove safety and performance
- Declaration of compliance with National Centre for Medical Devices Reporting (NCMDR) vigilance system
Ready to expand your medical device business from Algeria to Saudi Arabia?
How Operon Strategist Helps Algerian Manufacturers
Operon Strategist simplifies the complex SFDA registration journey for Algerian medical device manufacturers.
Our Key Services
- Regulatory Pathway Guidance – Assistance with classification and strategy
- MDMA Portal Support – Smooth application submission via SFDA system
- Technical Dossier Preparation – Compiling SFDA-compliant documents
- Authorized Representative Services – Helping appoint a Saudi-based agent
- QMS & ISO 13485 Compliance – Implementation support for required standards
- Post-Market Surveillance (PMS) – Guidance on vigilance systems and reporting
Looking for more guidance? Explore our related services:
FAQs
How long does SFDA medical device registration take?
On average, it takes 35 working days after complete submission, but timelines vary depending on device risk class and documentation quality.
Do Algerian manufacturers need a Saudi Authorized Representative?
Yes. All foreign manufacturers must appoint a local Saudi Authorized Representative to manage communication with SFDA.
What is the validity of SFDA registration?
SFDA medical device registration is valid for 3 years and must be renewed before expiry.