Medical Devices – Roles, Compliance & EDA Requirements
For foreign medical device manufacturers aiming to access the Egyptian market, appointing an Authorized Representative (AR) in Egypt is not optional—it is a mandatory requirement by the Egyptian Drug Authority (EDA). An AR ensures that your medical devices comply with EDA regulations, supports registration, and manages post-market activities.
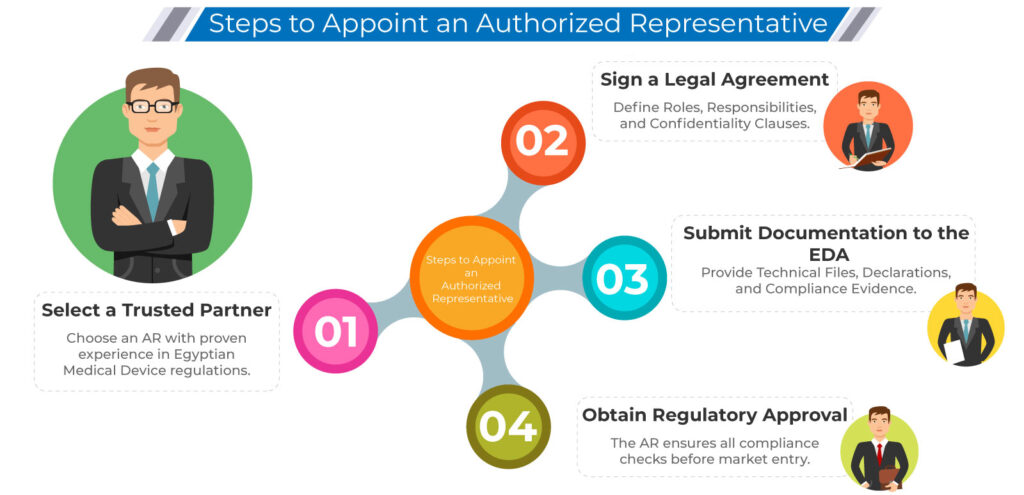
Choosing the right Authorized Representative is key to smooth market entry, faster approvals, and ongoing compliance.
Let's Grow Your Business Together Name
What is an Authorized Representative in Egypt?
An Authorized Representative (AR) is a local legal entity that acts on behalf of foreign medical device manufacturers in Egypt. Their main role is to:
Ensure compliance with EDA medical device regulations
Represent manufacturers before the Egyptian Drug Authority
Oversee registration, renewals, and technical documentation
Act as the primary contact point for authorities and local stakeholders
Why Do You Need an Authorized Representative in Egypt?
Foreign manufacturers without a local presence in Egypt must appoint an AR to meet EDA’s regulatory requirements. An Authorized Representative helps with:
Regulatory Compliance – Managing EDA registration, device approvals, and technical file submissions
Liaison with Authorities – Acting as the official communication channel with the EDA
Post-Market Surveillance – Handling vigilance reporting, recalls, and corrective actions
Labeling & Translation – Ensuring Arabic labeling and documentation compliance
Documentation Management – Preparing and maintaining dossiers, renewals, and amendments
Key Requirements for an Authorized Representative in Egypt
To serve legally as your AR, the entity must:
Have a registered office in Egypt
Be knowledgeable in EDA regulations and medical device directives
Handle technical file submissions, renewals, and compliance audits
Possess experience in post-market vigilance, complaints, and recalls
Responsibilities of an Authorized Representative After Device Approval
Even after EDA approval, the AR continues to support manufacturers by:
Managing Renewals & Amendments – Keeping registrations active and updated
Post-Market Vigilance – Coordinating recalls, complaints, and incident reporting with the EDA
Compliance Monitoring – Updating manufacturers on evolving Egyptian regulations
Ongoing Support – Acting as a long-term compliance partner in Egypt
How Operon Strategist Supports as Your Authorized Representative in Egypt?
At Operon Strategist, we serve as a trusted Authorized Representative for medical device manufacturers entering Egypt. With our expertise in EDA regulations and global compliance standards, we provide:
End-to-end EDA registration and compliance management
Preparation and submission of regulatory documentation & technical files
Full support in post-market surveillance, vigilance, and recalls
Guidance in ISO 13485 and CE Mark compliance for global market readiness
Turnkey consulting services for medical device manufacturing plants in Egypt
With our proven experience and strong industry network, we ensure your medical devices enter and stay in the Egyptian market smoothly and compliantly.
Your Local Partner in Egypt for Medical Device Success
Frequently Asked Questions (FAQs)
Yes, foreign manufacturers without a physical office in Egypt must appoint an AR to comply with EDA requirements.
In some cases, yes, but appointing a dedicated regulatory AR ensures better compliance and avoids conflicts of interest.
Typically, a technical file, labeling documents, free sale certificates, ISO certificates, and product details are required.
Timelines vary depending on the device class, but having an experienced AR can significantly reduce approval delays.
Because we combine regulatory expertise, ISO & CE consulting, and turnkey project support, making us a one-stop partner for compliance and market access.