Why Medical Device Classification Matters in Egypt?
When entering Egypt’s healthcare market, regulatory compliance is a critical step for medical device manufacturers. The Egyptian Drug Authority (EDA) oversees the classification, registration, and approval of medical devices, with strict protocols to ensure patient safety and device effectiveness.
At Operon Strategist, we’ve supported numerous manufacturers through every stage of regulatory approval — from medical device classification under EDA regulations to facility readiness, validation documentation, and cleanroom qualification. This guide explains how classification works, the risk categories involved, and how our tailored consulting services help simplify the entire process.
Let's Grow Your Business Together Name
What Is Medical Device Classification Under EDA Regulations?
Medical device classification under EDA regulations is a structured risk-based system that categorizes devices based on their intended use, patient interaction, and potential hazards. The classification directly impacts:
- The EDA registration process for medical devices
- Required documentation and risk management plans
- Conformity assessments and clinical evaluations
The system is broadly aligned with global frameworks like EU MDR and US FDA while addressing specific regulatory and operational needs unique to medical device regulations in Egypt.
How Are Medical Devices Classified in Egypt?
| Class | Risk Level | Examples |
|---|---|---|
| Class I | Low | Stethoscopes, surgical dressings |
| Class IIa | Low to medium risk | Dental fillings, surgical gloves |
| Class IIb | Medium to high risk | Infusion pumps, ventilators |
| Class III | High risk | Pacemakers, implantable devices |
Key Factors Influencing Medical Device Classification
The Egyptian Drug Authority (EDA) considers multiple criteria when classifying devices:
- Intended medical use
- Mode of operation and duration of body contact
- Level of invasiveness
- Whether the device delivers medicinal substances
- If the device is sterile, reusable, or implantable
Accurate Egypt medical device risk classification is crucial for determining the applicable registration pathway and technical documentation requirements.
EDA Medical Device Registration Process Overview
After classification is confirmed, manufacturers must navigate the EDA medical device registration Egypt process, which involves:
- Determining the device’s risk class
- Preparing a comprehensive regulatory dossier
- Ensuring plant, cleanroom, and manufacturing layout readiness (if applicable for local production)
- Completing conformity assessments, risk management documentation, and validation protocols
- Submitting to EDA and obtaining market authorization
At Operon Strategist, we support clients through every stage of this process — from dossier preparation to risk management planning, validation documentation consulting, and turnkey project support for new medical device facilities in Egypt.
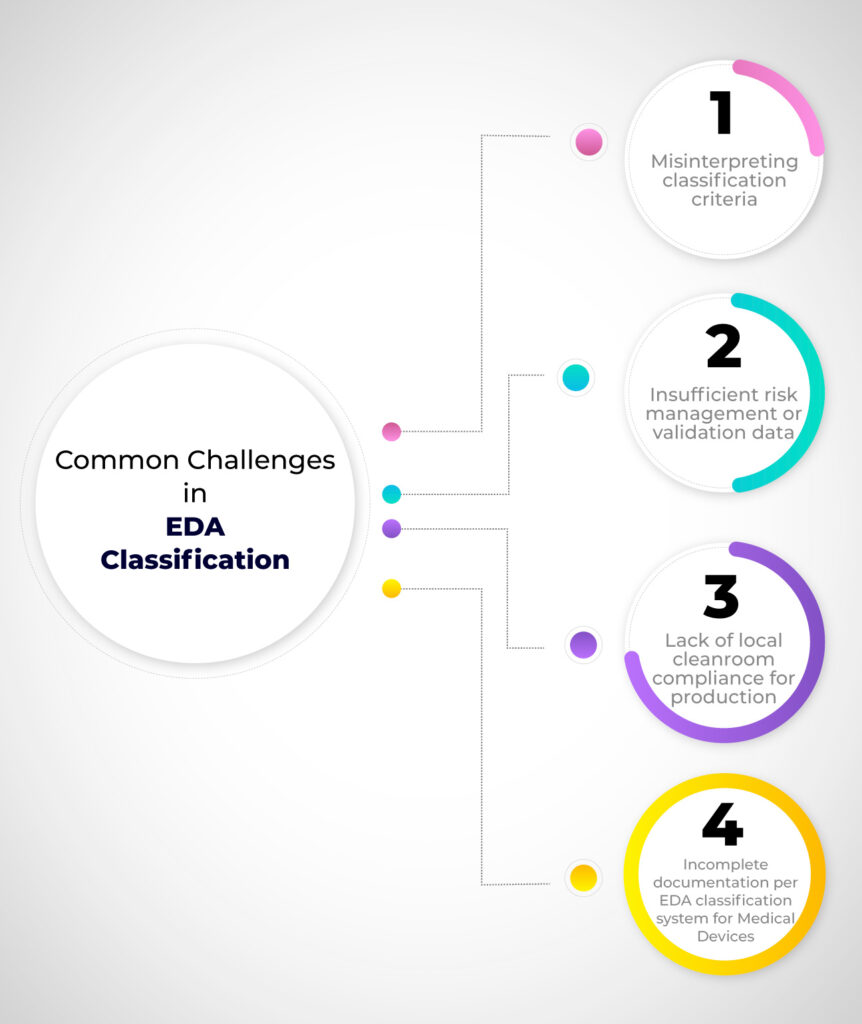
Why Is Accurate Classification Essential?
An incorrect classification can result in:
- Regulatory delays and rejection
- Additional costs for repeat testing or documentation
- Prolonged market entry timelines
To avoid such risks, partnering with an experienced regulatory team ensures classifications are accurate and fully compliant with medical device regulations in Egypt. Our consultants also help clients address related operational requirements like ISO 13485 implementation, CE marking for international market access, and FDA 510(k) submissions for US exports.
Operon Strategist’s Role in Supporting Medical Device Companies
At Operon Strategist, our consulting services are designed to simplify the complexities of medical device classification under EDA regulations and related processes. Our Egypt-based services include:
- Classification review and regulatory strategy
- Full EDA registration dossier preparation
- Turnkey project consulting for new or upgraded medical device plants
- Validation documentation for cleanrooms, equipment, and processes
- Assistance with ISO 13485 quality system implementation
- Risk management file development per EDA and international standards
- Guidance on CE Marking and MDSAP for multi-market readiness
- Cleanroom qualification and validation consulting
By combining regulatory expertise with operational readiness solutions, we ensure our clients meet all medical device regulations in Egypt efficiently.