Medical Device Design Consultants – Documentation & Development
Understanding Medical Device Design and Development
Medical device design and development is a structured, multistage process that requires careful planning, significant investment, and regulatory precision. Manufacturers must meet strict requirements from global regulators like the US FDA, EU MDR, and EDA (Egyptian Drug Authority) to ensure that devices are safe, effective, and backed by complete documentation.
As experienced Medical Device Design Consultants, Operon Strategist helps device makers transform innovative ideas into compliant, market-ready products. From ideation to validation, our consulting ensures your design meets quality standards, regulatory requirements, and patient safety expectations while minimizing risks of product recalls and market failure.
Let's Grow Your Business Together
Why Choose Medical Device Design Consultants?
Design and development documentation is not just paperwork — it is the foundation of regulatory approval. Without comprehensive design documentation, manufacturers may face delays, non-compliance, or permanent barriers to market entry.
Our role as Medical Device Design Consultants includes:
Establishing design controls in line with FDA 21 CFR Part 820.30 and ISO 13485:2016.
Documenting every stage of the design process from feasibility to validation.
Supporting compliance with international regulatory requirements.
Reducing risks of design flaws, safety issues, and costly recalls.
By partnering with consultants early, manufacturers can streamline development, reduce risks, and ensure faster approvals.
Global Market Outlook
The medical device design and development industry continues to expand, driven by the rising demand for digital health solutions, minimally invasive devices, and AI-powered technologies. Regulators worldwide are focusing on human factors engineering, SaMD (Software as a Medical Device) validation, and cybersecurity-first design.
This makes specialized Medical Device Design Consultants crucial for manufacturers aiming to achieve international success.
What is Medical Device Design?
As per the USFDA regulatory norms, it is the first step towards creating a medical device for the specific intended use. While designing the device the device process should focus on Market need, intended impact and safety of the end user. Avoiding any one of the said things may lead to financial loss or product recall.
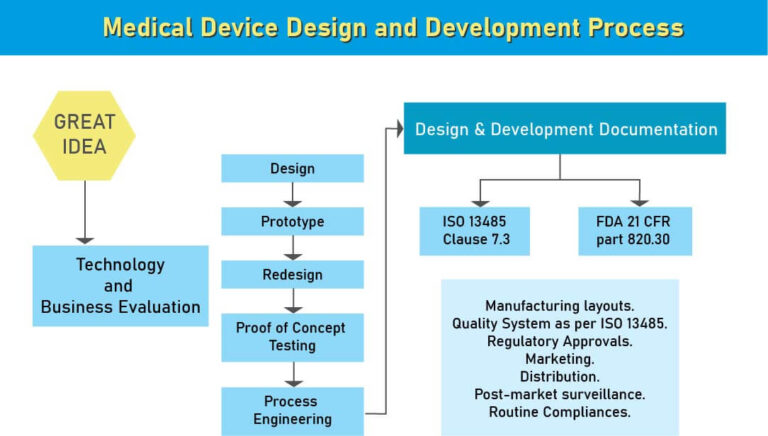
Medical Device Design Consultants’ Step-by-Step Process
Our consulting covers the entire product lifecycle with documentation support at every stage:
Feasibility – Market research, regulatory assessment, and clinical viability studies.
Planning – Developing a regulatory strategy, resource planning, and design roadmap.
Design & Development – Concept design, risk management, usability engineering, and prototyping.
Verification – Ensuring the design meets specifications through testing and analysis.
Validation – Confirming the device performs safely and effectively for its intended use.
Regulatory Submission Support – Preparing design documentation for FDA 510(k), CE Marking, EDA, and other global approvals.
By the later phases, the design funnel narrows, ensuring the final product is safe, compliant, and ready for manufacturing.
Design History File (DHF) in Medical Device Development
A Design History File (DHF) is a critical part of the medical device design process and is mandated by the US FDA under 21 CFR Part 820.30. It serves as a complete record of how a device was developed, including design inputs, outputs, reviews, verification, validation, and design transfer activities. A well-maintained DHF not only demonstrates regulatory compliance but also protects manufacturers from potential market setbacks and product recalls. As Medical Device Design Consultants, Operon Strategist assists manufacturers in creating, organizing, and maintaining DHFs that meet FDA and ISO 13485 requirements, ensuring smoother audits, inspections, and approvals.
From Concept to Compliance – Complete DHF Support
Emerging Trends in Medical Device Design
Medical device manufacturers must stay ahead of innovation and regulation. Our consultants integrate the latest trends into your design strategy, including:
AI-driven design & simulation – Faster prototyping and predictive risk analysis.
Digital twins – Virtual modeling to reduce costs and development time.
Human factors engineering – Improved usability and patient safety.
SaMD and cybersecurity-first design – Meeting FDA and MDR software requirements.
Sustainability in design – Eco-friendly materials and energy-efficient production.
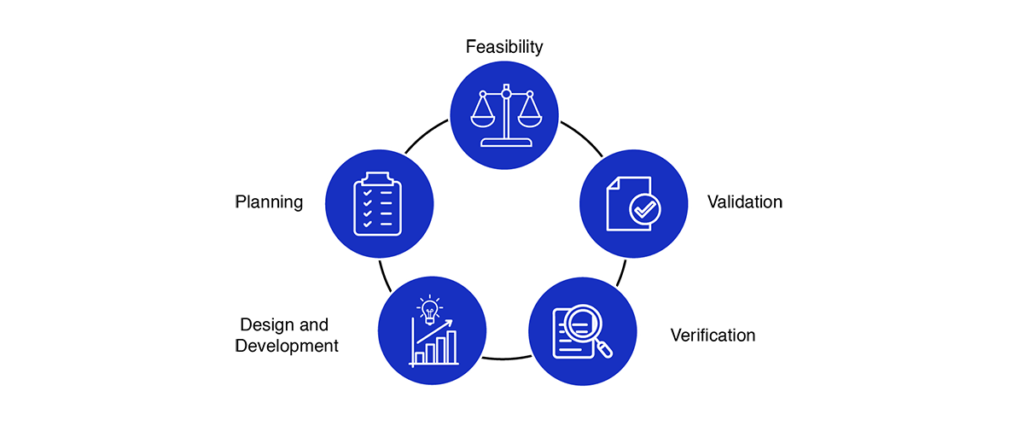
Medical Device Design Services & Product Development Process?
- Feasibility
- Planning
- Design and development
- Verification
- Validation
Operon Strategist’s Role as Medical Device Design Consultants
At Operon Strategist, we bring over a decade of proven expertise in guiding medical device manufacturers through the complexities of design control and regulatory compliance. Our team of seasoned consultants combines deep industry knowledge with hands-on experience across diverse global markets. We specialize in:
Validating tools and documentation required for Design History File (DHF) and Device Master Record (DMR) to meet FDA and ISO standards.
Assisting manufacturers with QMS implementation fully aligned with ISO 13485 and international best practices.
Offering expert guidance and practical support to manufacturers in Egypt, Costa Rica, India, South Africa, the US, and other global markets, tailored to local and international regulations.
Delivering cost-effective, error-free, and timely consulting services that help clients reduce risks, avoid compliance issues, and accelerate market entry.
With our industry network, regulatory expertise, and years of successful project execution, you can rely on Operon Strategist to drive your medical device from concept to compliance with confidence.
🚀 Build Stronger Design Documentation, Faster Approvals
FAQ'S
Design & Development Documentation refers to the controlled records required under medical device regulations—such as Design History File (DHF), design inputs, outputs, reviews, verification, validation, changes, and design transfer—to demonstrate compliance with design controls like US FDA 21 CFR 820.30.
A Design History File is a comprehensive record showing that your medical device was developed per an approved design plan, meeting applicable regulatory requirements under ISO 13485 or FDA 21 CFR Part 820.30.
The service addresses requirements of US FDA 21 CFR 820.30 Design Controls, ISO 13485:2016, and other regulatory standards for CE, UKCA and global approvals.
Deliverables typically include: a documented Design Plan; Design Inputs and Outputs; Review records; Verification & Validation protocols and reports; Change control documents; Design Transfer records; and the assembled Design History File (DHF).
Operon Strategist has over 10 years of experience in supporting medical device manufacturers with regulatory documentation, including DHF creation, risk management, validations and approvals for US, EU, India and other markets.