Intraocular Lens (IOL) Manufacturing Consultant: An Overview
Unlock Regulatory-Compliant Intraocular Lens Manufacturing Solutions for the UK and Global Markets
Intraocular lenses (IOLs) are transformative medical devices used to replace the eye’s natural lens, most commonly after cataract surgery. As the aging population increases across the UK and globally, so does the demand for high-quality, biocompatible IOLs. Establishing an IOL manufacturing unit in compliance with MHRA, EU MDR, and international standards requires precision, cleanroom environments, and a robust quality management system.
Operon Strategist, a leading global medical device regulatory consultant, offers end-to-end solutions for IOL manufacturing — from facility setup to CE marking support — helping UK-based manufacturers achieve fast and compliant market entry.
Let's Connect! Your Queries, Our Expertise!
What Are Intraocular Lenses (IOLs)?
Intraocular lenses are artificial lenses surgically implanted into the eye to restore clear vision, primarily after cataract removal. These lenses come in several types based on patient needs:
- Monofocal IOLs – For distance vision correction
- Multifocal IOLs – For both near and far vision
- Toric IOLs – For correcting astigmatism
Their design and material must ensure optimal optical performance, flexibility, and safety within the eye.
Intraocular Lens Manufacturing Process Overview
Establishing an IOL manufacturing unit involves several specialized processes, each aligned with quality standards:
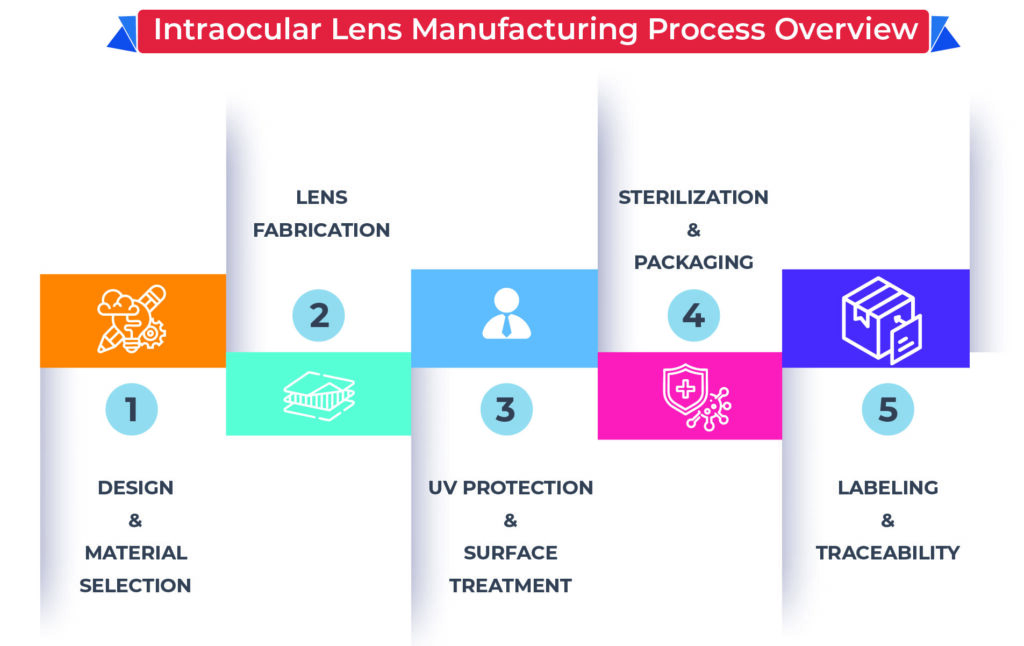
✅ Design & Material Selection
- CAD-based modeling for lens precision
- Medical-grade PMMA, hydrophobic acrylic, silicone
✅ Lens Fabrication
- Injection molding or lathe cutting for shaping
- Edge finishing for smooth implantation
✅ UV Protection & Surface Treatment
- Application of UV-blocking agents
- Enhanced biocompatibility and durability
✅ Sterilization & Packaging
- EtO or gamma radiation sterilization
- Packaged in ISO Class 7/8 cleanroom conditions
✅ Labeling & Traceability
Laser marking and batch coding in compliance with regulatory requirements
Key Materials & Machinery Used in IOL Manufacturing
Materials:
- PMMA (Polymethyl methacrylate)
- Silicone
- Hydrophobic/Hydrophilic Acrylic
Equipment:
- CNC lathes, injection molding machines
- UV curing units
- Laser marking systems
- Sterilizers (EtO/gamma)
- ISO-class cleanroom packaging systems
Regulatory Requirements for IOL Manufacturing in the UK
IOLs are classified as Class III medical devices, requiring stringent regulatory compliance:
- UK (Post-Brexit): UKCA Marking under MHRA regulations
- EU Market: CE Marking under EU MDR 2017/745
- USA: FDA 510(k) or PMA (for novel designs)
- India & Global Markets: CDSCO registration, ISO 13485 QMS
A comprehensive Technical File, risk management plan, clinical evaluation, and post-market surveillance system are essential components for UK and EU compliance.
Setting Up an Intraocular Lens Manufacturing Plant
Planning to launch an IOL manufacturing facility in the UK or serve international markets? Here’s what you need:
- Cleanroom Design: ISO Class 7/8 environment for aseptic production
- Manufacturing Plant Layout: Segregated zones for material movement, operator flow, and contamination control
- Validation Protocols: IQ/OQ/PQ for machines and utilities
- QMS Compliance: Implementation of ISO 13485:2016
- Regulatory Documentation: Device Master File, labeling standards, and registration
Start your IOL Manufacturing Project with Expert Guidance
Why Choose Operon Strategist as Your IOL Manufacturing Consultant?
At Operon Strategist, we specialize in turnkey consulting for ophthalmic device manufacturing, including IOLs. Our team has global regulatory experience and technical expertise to support UK manufacturers at every step:
- ✅ Turnkey IOL manufacturing plant setup
- ✅ Cleanroom design and HVAC systems
- ✅ CE Marking & UKCA Marking compliance consulting
- ✅ ISO 13485:2016 QMS implementation
- ✅ FDA 510(k) and CDSCO license support
- ✅ Equipment selection and process validation
- ✅ Regulatory audit preparedness
Whether you’re a UK-based startup or a multinational looking to expand, our consultancy services ensure your project is technically sound and globally compliant.
Ready to Start Your IOL Manufacturing Journey?
Let’s build a future-ready IOL manufacturing facility in the UK. Operon Strategist will support you from planning to regulatory approval, helping you reduce time-to-market and meet international standards.
📞 Contact Operon Strategist today for a free consultation.