MHRA IVD Registration: An Overview
Before placing medical devices or in-vitro diagnostic (IVD) devices on the market in Great Britain, manufacturers must comply with the MHRA IVD registration requirements. Governed by the UK Medical Devices Regulations 2002 (UK MDR 2002), this registration ensures that all medical devices and IVDs meet essential safety and performance standards.
In this article, we explain the MHRA registration process, who must register, the documentation needed, and what manufacturers should know before marketing their products in the UK.
Also, read our blog: The Ultimate Guide to MHRA Medical Device Registration
Let’s Connect! Your Queries, Our Expertise!
What is MHRA IVD Registration?
MHRA IVD registration is a mandatory notification process where medical device and IVD manufacturers register their products with the Medicines and Healthcare products Regulatory Agency (MHRA) before they are marketed in Great Britain (England, Scotland, and Wales). This process applies to all classes of medical devices and IVDs.
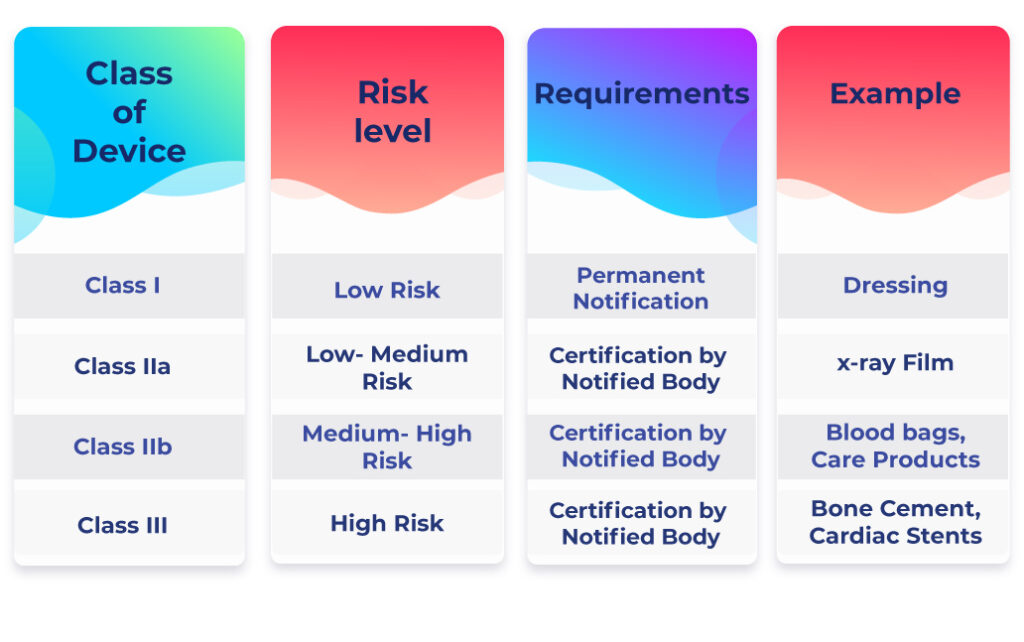
MHRA Classification of Medical Devices and IVDs
The MHRA (Medicines and Healthcare products Regulatory Agency) classifies medical devices and IVDs into the following categories:
- Class I, IIa, IIb, or III new medical devices and IVDs
- Class I, IIa, IIb, or III refurbished or rebranded devices
- IVDs undergoing performance evaluation
- Custom-made medical devices
- Systems or procedure packs that include at least one medical device
UK Medical Device Registration Requirements
- All medical devices, IVDs, custom-made devices, and procedure packs must be registered with the MHRA before being sold in Great Britain.
- UK-based manufacturers must apply for MHRA registration to place their devices on the market.
- Manufacturers outside the UK are required to appoint a UK Responsible Person (UKRP) to act on their behalf for MHRA registration.
- Importers based outside the UK must also:
- Appoint a UK Responsible Person
- Notify the relevant manufacturer of their intent to import
- Ensure the manufacturer or UKRP maintains their business details, including the location in Great Britain
- Distributors and suppliers are not required to register with the MHRA, but must handle only MHRA-registered devices.
What Types of Devices Require MHRA IVD Registration?
The following must be registered before entering the UK market:
- New Class I, IIa, IIb, III medical devices and IVDs
- Refurbished or relabelled medical devices
- IVDs undergoing performance evaluation
- Custom-made medical devices
- Procedure packs or systems with at least one medical device
Understanding the UK Medical Devices Regulations 2002
The UK MDR 2002 is the primary legal framework for regulating medical devices and IVDs in Great Britain. Originally enforced in 2002, it aligns with EU directives for active implantable devices and IVDs and is continuously updated for regulatory consistency.
Required Information for MHRA IVD Registration
To register a medical device or IVD, manufacturers must submit both manufacturer and device-specific details.
✅ Manufacturer Information
- Administrative contact (up to 15 people)
- Business type (e.g., sole proprietorship)
- Legal name and address as per device label
- UK Responsible Person designation letter (for overseas manufacturers)
✅ Device Information
- Applicable legislation and classification
- Basic UDI-DI and UDI-DI
- Device name, model/version, catalogue/reference number
- UK Approved or EU Notified Body (if applicable)
- GMDN code and description
Product features (e.g., sterility, contains latex, MRI compatibility)
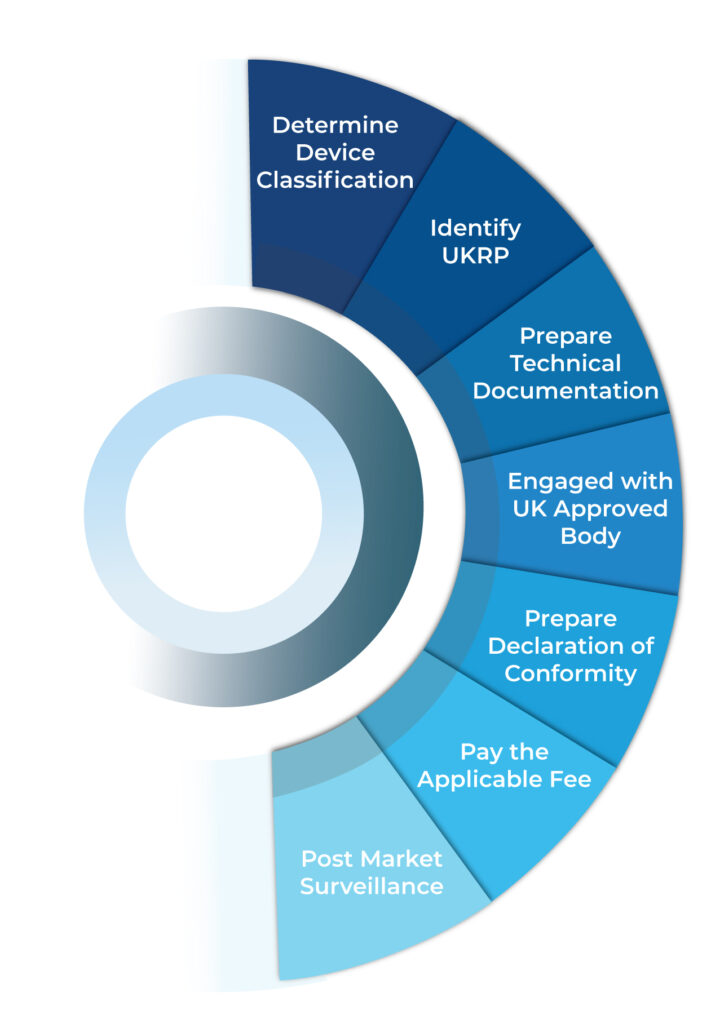
How to Register via the MHRA DORS Portal
- Create an account on the MHRA Device Online Registration System (DORS).
- Wait for account approval confirmation via email.
- Submit manufacturer and device data accurately.
- MHRA will review your submission within 5 working days.
- Additional documents may be requested (e.g., IFU, product images).
After completing registration with the MHRA, manufacturers receive authorization to sell and distribute their medical devices in the UK market. The registered devices are then listed in the MHRA’s public registration database.
⏳ MHRA Review Timeline
- Standard review time: 5 business days
- Extended if MHRA requests additional documentation
MHRA IVD Registration Renewal
To ensure up-to-date compliance, MHRA mandates regular updates:
- First renewal due 1 year after initial registration approval
- Subsequent updates every 2 years
- MHRA sends automated email reminders at 3, 2, and 1 month before the due date
- Renewal is currently free of charge
Final Thoughts
MHRA IVD registration is a critical first step for any manufacturer aiming to market medical devices or in-vitro diagnostics in Great Britain. While the process is straightforward, compliance with MHRA guidelines and timely renewals are essential for market access and business continuity.
By understanding the regulatory framework and following the correct procedure, manufacturers can ensure their products meet all legal requirements and avoid delays in market entry.
Get Expert Support for MHRA IVD Registration and UK Market Access
Need Help Navigating MHRA IVD Registration?
Getting your medical devices or IVDs registered in the UK can feel overwhelming, but you don’t have to do it alone. At Operon Strategist, we’re here to guide you every step of the way, from handling your MHRA application to acting as your UK Responsible Person.
Let’s make UK market entry smoother—reach out to our team today and let’s get started together!


