CE Marking for Medical Devices
Simple guidance to help you place your medical device in the EU market.
What CE marking means?
CE marking shows that a medical device meets the safety and performance rules set by the European Union under EU MDR 2017/745 or IVDR 2017/746. It is a legal requirement if you want to sell or distribute your device in the EU and EEA.
With CE marking, you confirm that your device is safe to use, works as intended, and follows all regulatory steps. This applies to all types of manufacturers, including UK, EU, US, and global companies that want to enter the European market.

Why CE marking matters?
CE marking helps you:
- Access 30+ countries in the EU and EEA
- Build trust with regulators, hospitals, and users
- Show that your device meets strict safety and quality rules
- Reduce compliance risks and market delays
- Keep your product competitive in Europe
Who needs CE marking
You must complete CE marking if you are:
- A UK manufacturer selling medical devices in the EU
- A global manufacturer exporting devices to Europe
- An authorised representative acting on behalf of a non-EU company
- A distributor or importer placing devices on the EU market
The CE Marking Process for Medical Devices in Europe
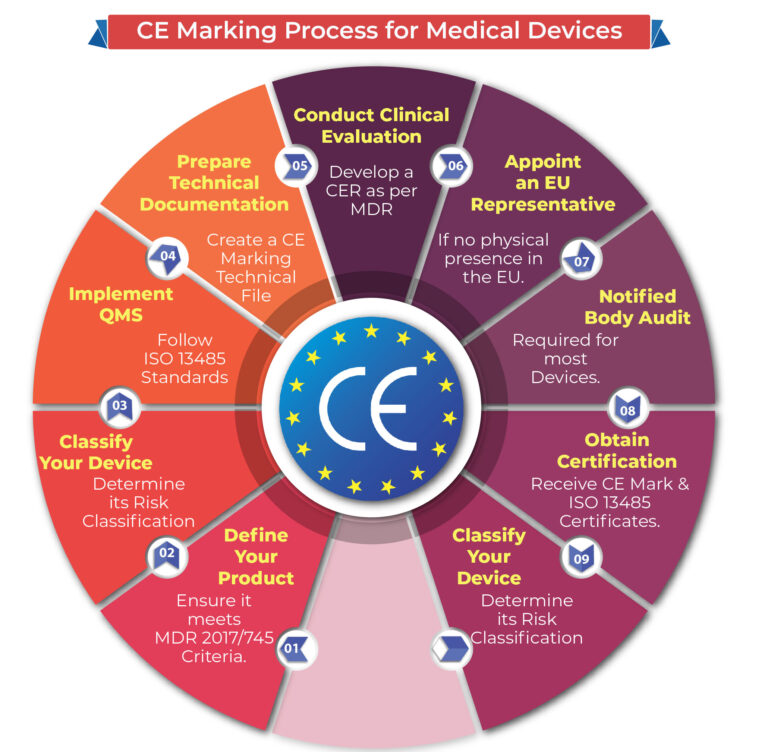
Compliance with the EU Medical Devices Regulation (MDR 2017/745) requires meeting specific standards of performance, quality, safety, and efficacy. The CE marking process generally includes:
- Defining Your Product: Confirm that your product meets the definition of a medical device per the MDR.
- Device Classification: Determine the classification of your device.
- Quality Management System (QMS): Implement a QMS, commonly ISO 13485, applicable to your device.
- Technical Documentation: Prepare a CE Marking Technical File or a Design Dossier.
- Clinical Evaluation: Develop a Clinical Evaluation Report (CER) as per MEDDEV 2.7/1 rev4 and MDR.
- Authorized Representative: If you have no physical location in the EU, appoint a European Authorized Representative (EC REP).
- Notified Body Audit: Your QMS and Technical File/Design Dossier must be audited by a Notified Body, unless your device is Class I, non-sterile, and non-measuring.
- Certification: Obtain CE Marking and ISO 13485 certificates from your Notified Body.
- Declaration of Conformity (DoC): Draft a DoC stating your device’s compliance with the MDR.
Documents needed for CE marking
The technical file usually includes:
- Device description and intended use
- Design details
- Risk management file
- Clinical evaluation report
- Performance testing
- Biocompatibility evidence
- Electrical and safety testing
- Sterilisation validation (if applicable)
- Software documents (for SaMD or software-driven devices)
- Labelling and IFU
- PMS and PMCF plans
- QMS records
We help you prepare each part in a simple, organised way.
Streamline Your EU Technical Documentation with Operon Strategist
Operon Strategist offers comprehensive services to help you secure CE marking, including:
- Product classification assistance
- Verification of standards and testing requirements
- Compilation or review of Technical Files or Design Dossiers
- Review of marketing materials, labeling, and user manuals for compliance
- Essential Requirements compliance verification
- Clinical Evaluation Report preparation
- Implementation and maintenance of quality systems (ISO 13485)
- European Authorized Representative services
- Risk assessment and management (ISO 14971)
- Development of vigilance and post-market surveillance procedures
To explore how Operon Strategist can support your CE marking journey, contact us at enquiry@operonstrategist.com or via WhatsApp.
Frequently Asked Questions about CE Marking
How long is a CE certificate valid?
CE certificates issued by Notified Bodies are generally valid for three years, but for some high-risk devices, the validity may only be one year. Your certification status is contingent upon maintaining your quality system certification.
Our device already has CE Marking. How does MDR 2017/745 affect our status?
The MDR came into full effect in May 2021. Certificates issued before the MDR’s implementation have a maximum validity of five years but will expire four years after the MDR comes into force.
Who issues my CE Marking certificate?
Notified Bodies issue CE certificates for Class I (provided sterile or has a measuring function), Class IIa, IIb, III medical devices, and certain IVDs. Self-certification is allowed for non-sterile, non-measuring Class I devices and general/other IVDs.
Does the Notified Body name appear on my product labeling?
The Notified Body’s four-digit NB number appears under the CE Mark symbol on your labeling, not their name.
Ensure smooth and compliant entry into the European market. Contact Operon Strategist today to learn how we can assist you in obtaining CE marking for your medical device.
Simplify Your CE Mark Journey
Get expert guidance on CE marking for medical devices. From classification to clinical evaluations, technical documentation, and EC REP services, we handle it all. Ensure compliance with EU MDR seamlessly.