Are you planning to launch your medical device in the UK? Understanding the MHRA medical device registration process is crucial for smooth market entry. In this guide, we’ll break down the registration process, costs, timelines, and key requirements to help you achieve compliance effortlessly.
What is MHRA Medical Device Registration?
MHRA registration is a legal requirement for manufacturers looking to place medical devices and in vitro diagnostic (IVD) devices on the UK market. It ensures compliance with UK-specific regulations, helping to maintain safety and efficacy standards.
Who Needs MHRA Registration?
✅ UK-based manufacturers of medical devices and IVDs.
✅ Non-UK manufacturers through a designated UK Responsible Person (UKRP).
✅ Distributors and importers handling medical devices in the UK.
Also read our service page on: UKCA for medical devices
Let's Grow Your Business Together
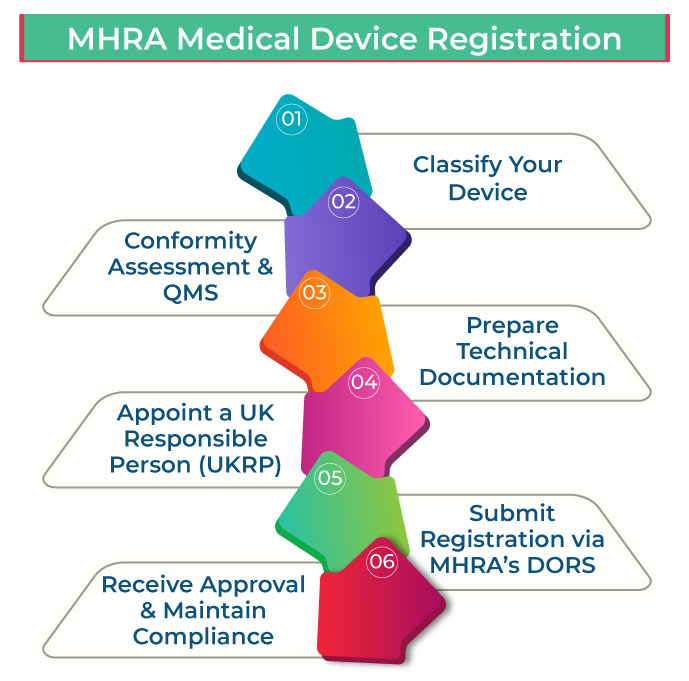
Step-by-Step Guide to MHRA Medical Device Registration
1️⃣ Classify Your Device
Your device classification determines the regulatory pathway. MHRA follows the UK MDR 2002 (as amended) for classification, similar to EU MDR rules:
- Class I, IIa, IIb, III (Medical Devices)
- A, B, C, D (IVDs)
Pro Tip: Higher-risk devices require certification from a UK Approved Body (UKAB) before registration.
2️⃣ Conformity Assessment & Quality Management System (QMS)
- Implement a Quality Management System (QMS) in compliance with ISO 13485.
- Choose a conformity assessment route based on device classification.
3️⃣ Prepare the Technical Documentation
This includes:
✔ Device description & classification rationale
✔ Risk assessment & clinical evaluation
✔ Instructions for use & labeling
✔ Performance testing & validation reports
4️⃣ Appoint a UK Responsible Person (UKRP) (For Non-UK Manufacturers)
A UKRP is mandatory for foreign manufacturers to represent their devices in the UK. They are responsible for:
✔ Registering the device with MHRA.
✔ Ensuring compliance with UK MDR 2002 regulations.
✔ Communicating with regulatory authorities on your behalf.
5️⃣ Submit Registration via MHRA’s Device Online Registration System (DORS)
- Pay the £240 registration fee.
- Submit the technical documentation and conformity assessment certificates.
- MHRA typically reviews applications within five business days, but delays may occur.
6️⃣ Receive Approval & Maintain Compliance
Once registered, your device will appear in the MHRA’s public database. Keep up with:
✔ Annual registration renewals (initial renewal after 1 year, then every 2 years).
✔ Post-market surveillance (PMS) and reporting any adverse incidents.
MHRA Medical Device Registration Fees & Timelines
MHRA Registration Fee: £240 per submission (up to 100 devices in one application).
Review Time: 5 business days, but can be extended if MHRA requests additional information.
Renewal: 1 year after initial registration, then every 2 years.
What Happens After MHRA Approval?
Once your medical device is successfully registered, you must:
✔ Monitor compliance with MHRA regulations.
✔ Ensure product traceability for post-market surveillance.
✔ Renew registration within the required timeframe.
Ready to Register Your Medical Device in the UK?
Why Choose Operon Strategist for MHRA Registration?
At Operon Strategist, we specialize in:
✔ Hassle-free MHRA registration for medical device manufacturers.
✔ Expert guidance on UKCA marking & regulatory compliance.
✔ Comprehensive support from technical documentation to market approval.
Get expert consultation today! Contact us to simplify your MHRA medical device registration.
Talk to Our Experts and accelerate your UK market entry!
FAQs: MHRA Medical Device Registration
1. Do all medical devices need MHRA registration?
Yes, all medical devices and IVDs must be registered with MHRA before being placed on the UK market.
2. Can non-UK manufacturers register directly?
No, non-UK manufacturers must appoint a UK Responsible Person (UKRP) for registration.
3. How long does MHRA registration take?
The standard review time is 5 business days, but delays can occur if additional information is requested.
4. What is the difference between UKCA and CE marking?
UKCA marking is required for devices in Great Britain (England, Scotland, Wales), while CE marking applies to EU markets.
5. How often do I need to renew my MHRA registration?
The first renewal is after 1 year, and subsequent renewals are required every 2 years.