Introduction: Understanding UKCA Marking for Medical Devices
With the UK’s exit from the EU, medical device manufacturers now need to comply with the UK Medical Devices Regulations 2002 (UK MDR 2002) to place their products on the market in England, Wales, or Scotland. A key part of this compliance is affixing the UK Conformity Assessed (UKCA) mark, which proves your device meets safety and performance standards as required by UK law. In this blog, we’ll explore the UKCA marking requirements for medical devices, the process to obtain the mark, and the obligations of manufacturers.
Also, read our service page on: UKCA marking for medical devices
Let's Connect! Your Queries, Our Expertise!
What Is the UKCA Mark and Why Is It Important?
The UKCA (UK Conformity Assessed) mark is the official symbol indicating that a medical device complies with UK MDR 2002. This mark is legally required for all devices placed on the market in England, Wales, and Scotland. It replaces the CE mark in Great Britain, though CE marking is still accepted in Northern Ireland, where different regulations apply.
Failure to comply with UKCA requirements may result in:
- Enforcement actions from the Medicines and Healthcare products Regulatory Agency (MHRA)
Please read our blog on: The Ultimate Guide to MHRA Medical Device Registration
- Civil or criminal penalties
- Removal of non-compliant or unsafe products from the market
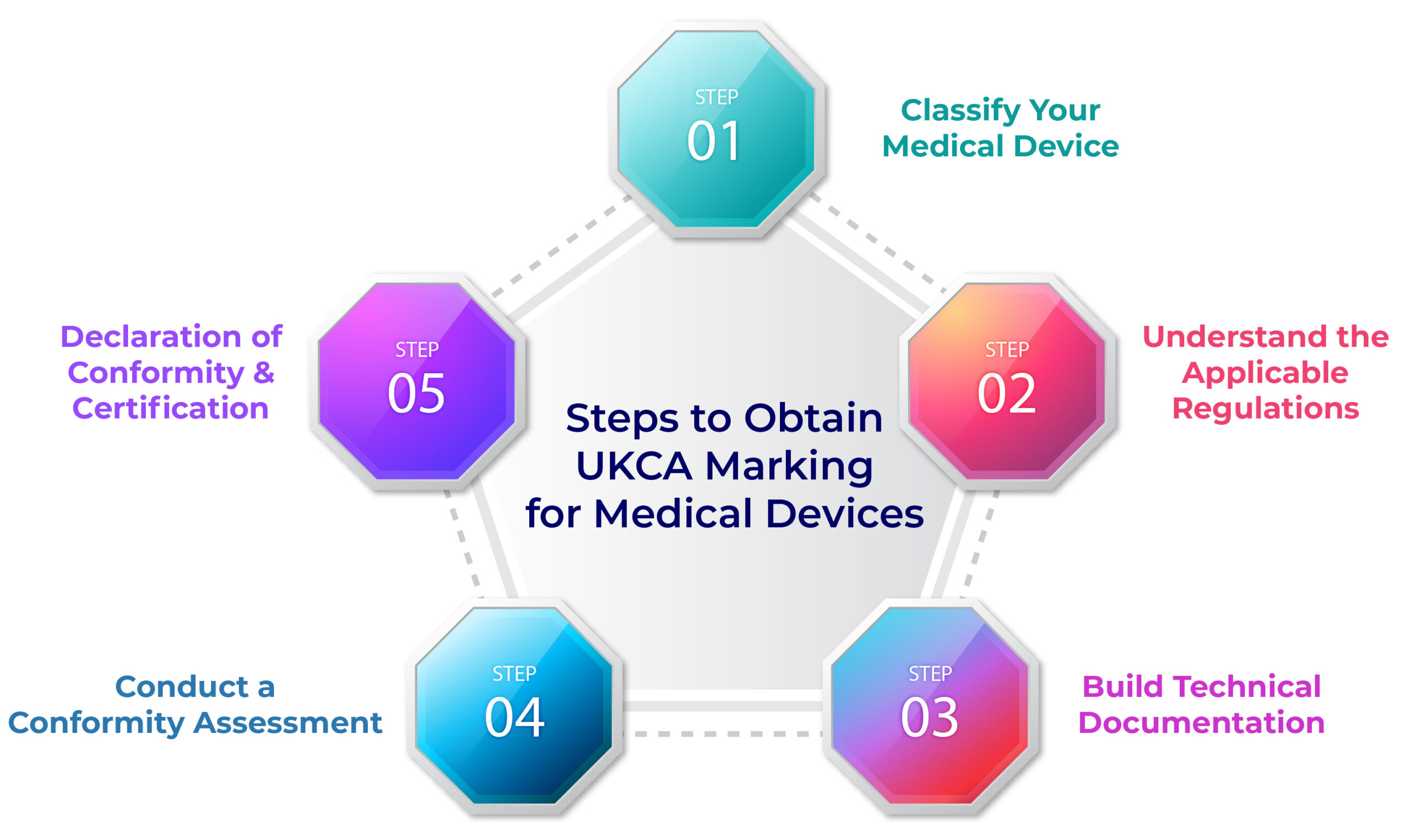
Steps to Obtain UKCA Marking for Medical Devices
To successfully place your medical device on the Great Britain market, follow these steps:
1. Classify Your Medical Device
Determine the risk class of your device under UK MDR 2002:
- General medical devices (Part 2)
- Active implantable medical devices (Part 3)
- In vitro diagnostic (IVD) devices (Part 4)
If unsure about classification, refer to MHRA’s official guidance on determining medical device status.
2. Understand the Applicable Regulations
You must identify which parts of the UK MDR 2002 apply to your device based on its intended use and classification. Each device class has specific essential requirements for safety and performance that must be met.
3. Build Technical Documentation
Create a technical file that clearly outlines:
- Product specifications
- Risk management activities
- Design and manufacturing processes
- Clinical evaluation or performance data
- Quality Management System (QMS) details
Most of this documentation can be derived from a compliant ISO 13485:2016 QMS. Establish your QMS early to ensure systematic and efficient documentation.
4. Conduct a Conformity Assessment
- Class I devices: Manufacturers can self-declare conformity (excluding those with a sterile or measuring function).
- Higher-risk devices (Class IIa, IIb, III, AIMD, IVDs): Require assessment from a UK-approved body.
Use the UK government’s list to find a qualified approved body for your device type.
5. Declaration of Conformity & Certification
After successfully passing the conformity assessment:
- Prepare a UK Declaration of Conformity
- Obtain the certificate of conformity from your approved body
Final Steps: Applying the UKCA Mark
Once certification is granted:
- Affix the UKCA mark visibly on your device
- Include the approved body identification number (for higher-risk devices)
- Register the manufacturer and device with the MHRA before placing it on the market
If the device undergoes significant changes post-market, a reassessment is required to ensure continued compliance.
Stay Compliant and Informed
Compliance with UKCA marking requirements is a legal obligation that ensures product safety and protects patient well-being. Non-compliance can result in significant legal and financial consequences. It’s essential for manufacturers to stay updated on MHRA regulations and ensure that all technical, clinical, and quality documentation is in place.
How Operon Strategist Can Help
Operon Strategist supports medical device manufacturers with:
- UKCA conformity documentation preparation
- Device classification and regulatory strategy
- QMS setup (ISO 13485)
- Liaising with UK-approved bodies
MHRA registration support
Get Your Device UKCA-Certified With Expert Guidance From Operon Strategist
Need Help Navigating UKCA Marking?
Let Operon Strategist guide you through every step of the UKCA certification process.
Contact us today to ensure your medical devices meet UK market regulations smoothly and efficiently.