Medical Device Validation process Consultant :
What is medical device Validation?
Medical Device Process Validation is a process of establishing documentary evidence demonstrating that a procedure, process or activity carried out in production maintains the desired level of compliance at all stages. In straightforward words, the interaction approval for a medical device is the assortment and assessment of information, from the cycle configuration stage till the creation, as it gives logical proof that the cycle is able to do reliably giving quality items. In simple words, the process validation medical device is the collection and evaluation of data, from the process design stage till the production, as it gives scientific evidence that the process is capable of consistently providing quality products.
What is the difference between process verification and validation?
According to USFDA process validation means establishing the objective evidence that a process consistently meets the products Predetermined specifications. Verification process means verifying the quality of product but testing every single device is impractical, and process validation comes into picture. The process validation completes the quality assurance need. As an ISO 13485 medical device consultant We know the guidelines provided by the regulatory authority and we help manufacturers to implement them correctly.
Medical Device Process Validation Services :
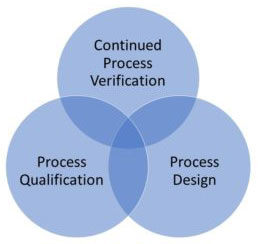
Medical Device Process Validation is divided into the following sub-sections:
- HVAC validation
- Equipment validation
- Process validation
- Facilities validation
- Cleaning validation
- Analytical method validation
- Personnel validation
- Packaging validation
- Computer system validation
Why choose Operon Strategist as validation process consultant?
We have validation experts who can guide you in design qualification, site acceptance, guidance for preparing FDA medical device process validation protocols & reports, provide validation consulting for facility and utility qualification, medical device manufacturing and packaging validation, design validation, etc.
For more details on validation services please contact us. Our global presence adds the experience of working with various regulatory bodies, if we particularly talk about UK region our satisfied customer base inspires us to provide more and more quality services.
We also provide medical device consultation for the USA, Costa Rica, the UK, Oman, South Africa, Saudi Arabia, & Egypt. Contact us now for a free consultation.
