Introduction to Disposable Syringe Manufacturing:
Did you know that Oman’s healthcare sector is rapidly growing, creating a demand for high-quality medical devices like disposable syringes? Oman’s medical device manufacturing industry presents lucrative opportunities for businesses looking to set up manufacturing units.
In this guide, we’ll explore:
- The manufacturing process of disposable syringes
- The raw materials required
- Regulatory compliance and approval process
- Challenges and how to overcome them
Let’s dive into establishing a successful syringe manufacturing unit in Oman!
Looking For a Medical Device Regulatory Consultant?
Let’s have a word about your next project
1. Understanding Disposable Syringes
A disposable syringe is a single-use medical device used for injecting or withdrawing fluids. These syringes are widely used in:
- Hospitals & clinics
- Diagnostic labs
- Vaccination programs
- Home healthcare
Types of Disposable Syringes
- Luer Lock Syringes – Ensures a secure needle fit
- Luer Slip Syringes – Quick needle attachment
- Insulin Syringes – Used for diabetes care
- Safety Syringes – Prevent needle-stick injuries
The global disposable syringe market is growing at a CAGR of 5.6%, making it a profitable sector to invest in!
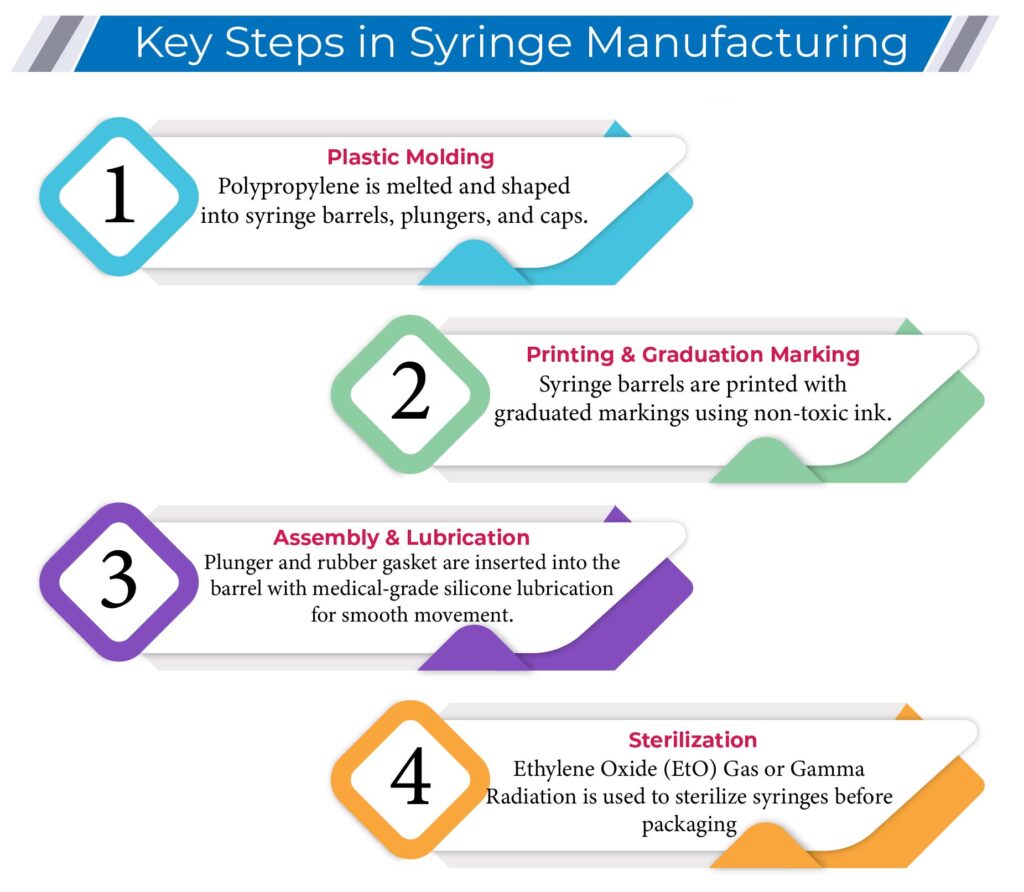
2. Key Steps in Syringe Manufacturing
3. Raw Materials & Equipment Required
Raw Materials
- Polypropylene (PP) – For barrels and plungers
- Rubber Gaskets – Ensures a secure plunger seal
- Medical-Grade Lubricant – For smooth syringe operation
- Needles & Stainless-Steel Cannulas
Essential Machinery
- Injection Molding Machine
- Printing Machine
- Assembly Line
- Sterilization Chamber
💡 Choosing high-quality raw materials improves product durability and compliance.
4. Regulatory Compliance in Oman
Manufacturers must comply with Oman’s Ministry of Health (MOH) regulations and international standards such as:
- ISO 13485:2016 – Quality Management System for medical devices
- ISO 10993 – Biocompatibility of medical devices
- GMP (Good Manufacturing Practices) compliance
- Import & Registration Requirements under Oman’s MOH
📌 Regulatory approval is crucial for exporting syringes to international markets like the GB, EU, US, and more.
5. Challenges & Solutions
Challenges
❌ High Initial Investment – Machinery & facility setup costs
❌ Regulatory Hurdles – Compliance with MOH & international standards
❌ Competition – Competing with established global brands
Solutions
✅ Financial Planning & Grants – Government incentives for local manufacturers
✅ Regulatory Consultants – Hiring experts like Operon Strategist for regulatory guidance
✅ Advanced Technology – Investing in automated production for cost efficiency
Overcoming these challenges can make Oman a key player in the syringe manufacturing sector!
Need Expert Guidance for Setting up a Syringe Manufacturing Unit in Oman?
6. Conclusion
✅ Oman’s syringe manufacturing industry is a promising venture with a growing demand in the healthcare sector.
✅ Following regulatory guidelines ensures compliance and export opportunities.
✅ Investing in high-quality materials and advanced technology enhances competitiveness.
🔴 Need help with regulatory approvals? Contact Operon Strategist for expert consultation!
What challenges do you foresee in setting up a syringe manufacturing unit?