Blood Collection Tubes Manufacturing and Regulatory Compliance in Oman
Blood collection tubes manufacturing in Oman is a growing opportunity driven by the demand for reliable diagnostic devices and self-sufficiency in healthcare. Operon Strategist helps investors and manufacturers set up compliant manufacturing units for blood collection tubes that meet ISO 13485, GMP, CE Marking, and Oman Ministry of Health (MOH) regulations. From feasibility study and plant setup to product development and regulatory approval, our experts support you at every step.
Want to take your blood collection tube manufacturing project to the next level? Contact Operon Strategist today for expert guidance on manufacturing plant layout design and turnkey solutions that can transform your blood collection tube manufacturing project.
Looking For a Medical Device Regulatory Consultant?
Let’s have a word about your next project
Why Invest in Blood Collection Tubes Manufacturing in Oman?
Oman’s Ministry of Health (MOH) has been actively encouraging local manufacturing of medical devices to reduce dependency on imports. This makes the country a strategic location for launching medical device production units like blood collection tubes.
Key Benefits:
- Increasing demand from hospitals, clinics, and diagnostic labs
- Reduced import reliance and logistics costs
- Government support for medical device manufacturing
- High ROI due to consistent demand
How Are Blood Collection Tubes Manufactured?
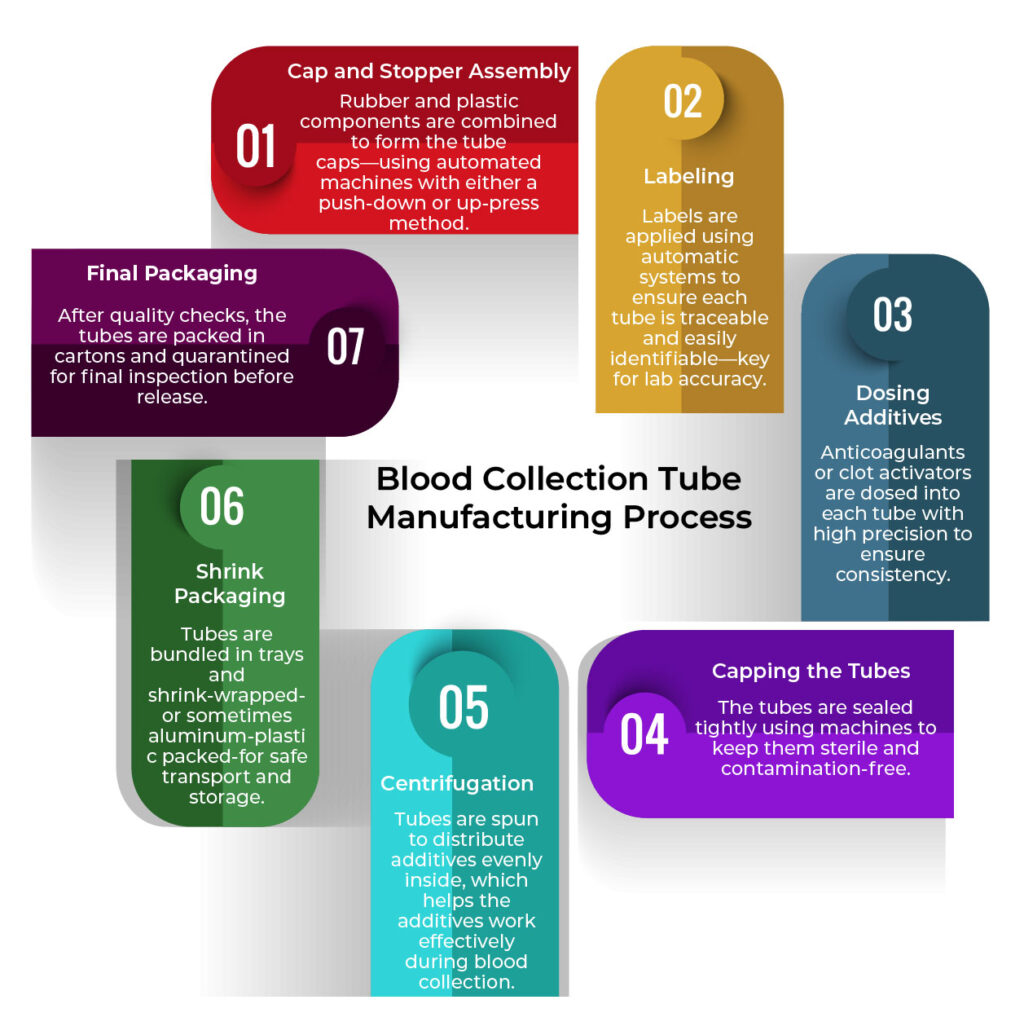
Manufacturing these tubes requires a clean, precise, and well-coordinated setup. Here are the 7 main steps:
- Cap and Stopper Assembly
Rubber and plastic components are combined to form the tube caps, using automated machines with either a push-down or up-press method. - Labeling
Labels are applied using automatic systems to ensure each tube is traceable and easily identifiable—key for lab accuracy. - Dosing Additives
Anticoagulants or clot activators are dosed into each tube with high precision to ensure consistency. - Capping the Tubes
The tubes are sealed tightly using machines to keep them sterile and contamination-free. - Centrifugation
Tubes are spun to distribute additives evenly inside, which helps the additives work effectively during blood collection. - Shrink Packaging
Tubes are bundled in trays and shrink-wrapped—or sometimes aluminum-plastic packed—for safe transport and storage. - Final Packaging
After quality checks, the tubes are packed in cartons and quarantined for final inspection before release.
Supporting Equipment & Processes
Aside from the main production line, here’s what else is involved:
- Tube Molding Machines
- Cleaning Stations for Components
- Sterilization Units, like gamma or EO sterilizers
- Automated or Semi-Automated Assembly Lines
Regulatory Compliance for Blood Collection Tubes in Oman
Medical devices like blood collection tubes are regulated in Oman under MOH guidelines. To enter the market, manufacturers must comply with:
- ISO 13485:2016 QMS for medical devices
- Good Manufacturing Practices (GMP)
- CE Marking (for export to EU)
- US FDA 510(k) (for US market, if applicable)
- Local MOH product registration
At Operon Strategist, we ensure your facility, process, and documentation meet all these regulatory benchmarks. We help prepare your Device Master File (DMF), risk management reports, sterilization validation, clinical evaluation (if needed), and product dossier for submission.
Our Services for Blood Collection Tubes Manufacturing in Oman
Operon Strategist provides end-to-end consulting services for setting up a fully compliant and operational blood collection tube manufacturing plant:
- Detailed project report (DPR)
- Regulatory & documentation support
- ISO 13485 QMS implementation
- Plant layout & HVAC cleanroom design
- Supplier, machine, and raw material sourcing
- Product development & prototyping
- Validation and sterilization guidance
- CE Marking and MOH registration
- Internal audits and training programs
Ready to Set Up Your Blood Collection Tubes Manufacturing Unit in Oman?
Why Choose Operon Strategist?
We are a trusted global consultant with years of experience in medical device turnkey projects, including for IVD and consumables like blood collection tubes. Our local understanding of Oman’s regulatory system, combined with global quality standards, makes us your ideal partner.
- Cost-effective project execution
- ISO & CE compliance experts
- Fast-track regulatory approvals
- Experienced in Middle East markets