IOL Cast Molding Technology: Transforming Intraocular Lens Manufacturing in Oman
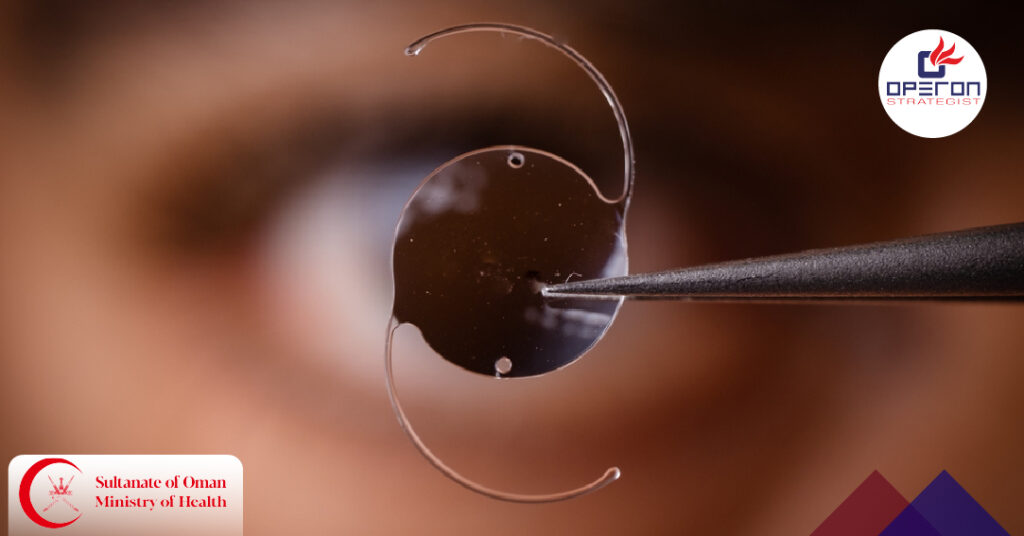
IOL Cast Molding Technology: Transforming Intraocular Lens Manufacturing in Oman IOL Cast Molding Technology is revolutionizing intraocular lens (IOL) manufacturing worldwide — and Oman is no exception. With cataract surgeries increasing in the region and growing demand for premium vision correction, ophthalmic device manufacturers are adopting cast molding to ensure precision, repeatability, and compliance with […]
IOL Cast Molding Technology: Transforming Intraocular Lens Manufacturing in Oman Read More »