Overview of Dental Implants Manufacturing Industry
Are you exploring the opportunity to enter the growing market of dental implants in Oman? With increasing demand for durable, biocompatible tooth replacement options, dental implants manufacturing presents a high-potential sector backed by evolving healthcare infrastructure and regulatory clarity.
This blog provides a comprehensive guide to manufacturing processes, materials used, compliance requirements, and how Operon Strategist supports you every step of the way.
Looking For a Medical Device Regulatory Consultant?
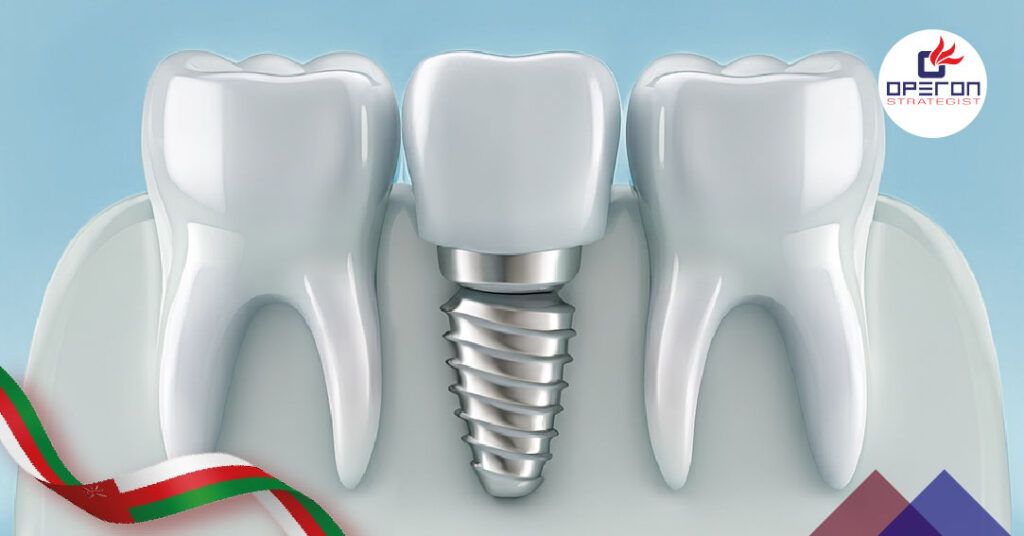
What Are Dental Implants?
Dental implants are artificial tooth roots, typically made from titanium, surgically inserted into the jawbone to support a crown, bridge, or denture. They offer a long-lasting, natural-looking solution for patients with missing teeth.
Each dental implant system generally consists of:
- Implant Fixture/Base: A screw-like titanium structure that fuses with the bone.
- Abutment (Connector): Connects the implant to the prosthetic.
- Prosthetic Crown: The visible, tooth-like component made from ceramic or zirconia.
Dental Implant Manufacturing Process
Creating precision-engineered dental implants requires specialized machinery and cleanroom conditions. Here’s a breakdown of the typical manufacturing process:
- CNC/VMC Machining
High-precision computer-controlled machines shape the implant fixture with exact specifications. Vertical machining centers (VMCs) enhance surface accuracy. - Sandblasting
This step roughens the surface of the implant to improve osseointegration with the bone. - Acid Etching
Implants undergo chemical etching to further enhance surface texture, promoting better tissue integration. - Anodizing
The implant is submerged in an electrolyte bath and charged with electricity to improve wear resistance, corrosion protection, and color coding. - Electropolishing
Removes microscopic burrs and gives a smooth, clean finish to reduce bacterial adhesion. - Ultrasonic Cleaning
Ultrasonic waves ensure thorough cleaning, eliminating residue and contaminants. - Laser Marking
Permanent identification markings are applied for traceability and regulatory compliance.
Materials Used in Dental Implants
Manufacturers must carefully select materials that ensure strength, corrosion resistance, and biocompatibility. The most widely used materials include:
- Titanium (Grade 4 or 5): Offers superior biocompatibility and osseointegration.
- Titanium Alloy (Ti-6Al-4V): Lightweight, durable, and corrosion-resistant.
- Zirconia: A ceramic material prized for aesthetics and suitable for patients allergic to metals.
- Stainless Steel: Sometimes used in temporary implants.
- Cobalt-Chromium (Co-Cr) Alloy: Less common but still biocompatible.
All materials must comply with ISO 10451:2010 and ISO 13485:2016 standards to ensure safety and performance.
Regulatory Compliance in Oman for Dental Implant Manufacturing
Dental implants are classified as Class C or D medical devices, depending on their complexity and risk. Manufacturers looking to establish operations or sell in Oman must comply with:
- Ministry of Health (MoH) – Oman medical device registration guidelines
- ISO 13485 Certification for Quality Management System (QMS)
- GMP (Good Manufacturing Practice) compliance
- CDSCO (India) or US FDA (for exports) regulatory approvals if applicable
- CE Marking for products intended for European markets
Working with expert consultants ensures faster approvals, avoids delays, and helps meet stringent inspection protocols.
Market Potential for Dental Implants in Oman
The dental implant sector in Oman is witnessing steady growth due to:
- Rising elderly population and age-related tooth loss
- Increased medical tourism and cosmetic dentistry demand
- Expanding number of dental clinics and surgical professionals
- Government investments in advanced dental care infrastructure
Now is the right time to invest in dental implants manufacturing in Oman—a future-ready sector.
Ready to Start Your Dental Implants Manufacturing Facility in Oman?
How Operon Strategist Can Help You
At Operon Strategist, we provide end-to-end consulting for dental implant manufacturing projects:
Regulatory Consulting
- ISO 13485, CE Marking, US FDA, and CDSCO compliance support
- Product classification and documentation for Oman’s Ministry of Health
- Technical file creation, risk management, and post-market surveillance setup
Turnkey Project Solutions
- Cleanroom design and layout for implant production
- Machinery selection and vendor coordination
- QMS documentation and internal audits
- Training of staff in ISO/GMP practices
Market Expansion Support
- Product registration assistance in Oman, GCC, India, and EU
- Branding and traceability strategy via UDI & laser marking
- Post-approval compliance monitoring