Introduction: Why Medical Device Registration in Oman Matters
Oman’s growing healthcare sector presents a high-potential market for global medical device manufacturers. To legally sell your products in the Sultanate, medical device registration in Oman is mandatory. Regulated by the Directorate General for Pharmaceutical Affairs and Drug Control (DGPA&DC) under the Ministry of Health (MoH), the country’s current framework allows for streamlined market access via a notification-based system.
In this comprehensive guide, we outline everything you need to know about registering medical devices in Oman in 2025, including classification, documentation, timeframes, and how Operon Strategist can help ensure full regulatory compliance.
Also, check out our service page on Medical Device Registration in Oman for detailed guidance and expert support.
Looking For a Medical Device Regulatory Consultant?
Let’s have a word about your next project
Who Regulates Medical Device Registration in Oman?
The DGPA&DC is the primary authority responsible for overseeing medical device registration in Oman. While Oman does not yet follow a rigid, standalone medical device regulatory law like the EU MDR or US FDA CFR 21, it currently operates under a notification system.
This approach accelerates approvals for devices already approved in markets such as:
- US FDA (510(k), PMA)
- EU CE (under MDR)
- Health Canada
- Australia (TGA)
- Japan (MHLW)
Medical Device Classification System
Oman does not maintain an exclusive classification system but instead recognizes classifications from:
- European MDR: Class I, IIa, IIb, III
- US FDA: Class I, II, III
- TGA, MHLW, Health Canada
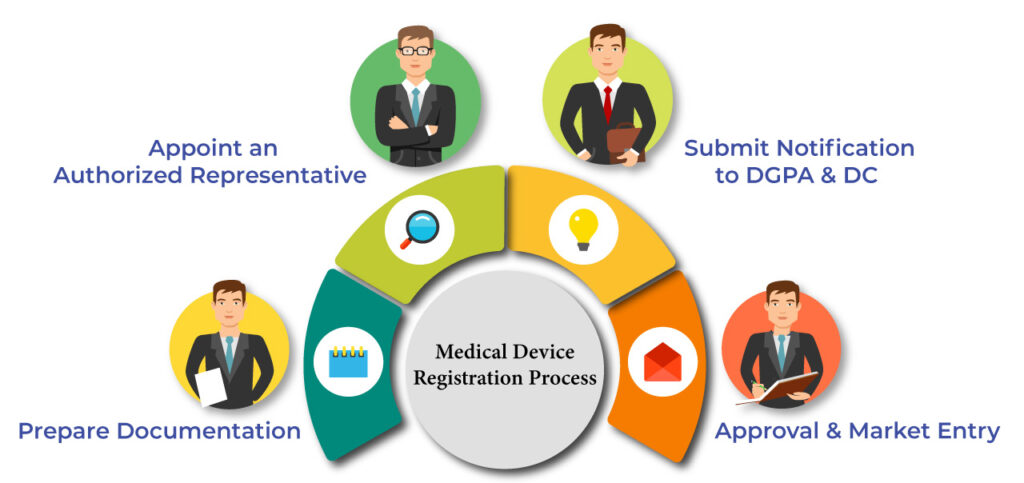
Medical Device Registration Process
Oman follows a notification system for medical devices, making the process faster and more efficient. Here’s a step-by-step breakdown:
Here’s how the medical device registration process in Oman works under the current notification system:
Step 1: Documentation Preparation
Compile a comprehensive dossier including:
- Product description & intended use
- CE Certificate / FDA Clearance
- Instruction for use (IFU)
- Labeling & packaging artwork
- ISO 13485 certification
Step 2: Appoint a Local Authorized Representative
A local representative in Oman is mandatory for all foreign manufacturers. This entity will:
- Submit documents to DGPA&DC
- Handle communications and queries
- Maintain regulatory compliance
Step 3: Submit Notification to DGPA&DC
Submit the notification file to the regulatory authority. If all documentation is satisfactory, the product gets listed in the Oman medical device registry.
Step 4: Market Approval & Launch
Upon approval, you receive market authorization to legally distribute your product in Oman.
Timeframe: Typically 3–4 months for approval, depending on dossier completeness.
License Validity & Renewal in Oman
- Validity Period: 5 years
- Renewal: Must begin at least 6 months before expiration
- Updated documents may be required (e.g., revised labeling, design changes)
Role of the Local Authorized Representative
The Authorized Representative is a legal and regulatory liaison in Oman. Their responsibilities include:
- Dossier submission & renewal applications
- Handling post-market surveillance & incident reporting
- Managing DGPA&DC queries
- Ensuring labeling and packaging compliance
Ready to Register Your Medical Device in Oman?
How Operon Strategist Helps with Medical Device Registration in Oman
We offer end-to-end regulatory consulting services for manufacturers aiming to register and market medical devices in Oman. Our expert team ensures complete compliance with local and international standards through:
- Dossier compilation & regulatory review
- Appointing a reliable authorized representative in Oman
- DGPA&DC submission and communication support
- Post-approval support & license renewal assistance
- ISO 13485 & CE Mark compliance consulting
Beyond Oman, Operon Strategist also provides global regulatory assistance including FDA medical device registration for U.S. market entry and CDSCO registration for medical devices to help manufacturers access the Indian market. Our proven expertise ensures smooth approvals, faster market access, and sustained compliance across multiple regulatory jurisdictions.
FAQs
Is medical device registration mandatory in Oman?
Yes. Medical device registration in Oman is mandatory for all devices intended to be imported, marketed, or distributed in the Sultanate. Products must be notified and approved by the Directorate General for Pharmaceutical Affairs and Drug Control (DGPA&DC) under the Ministry of Health before market entry.
Which authority regulates medical devices in Oman?
Medical devices in Oman are regulated by the DGPA&DC (Directorate General for Pharmaceutical Affairs and Drug Control), functioning under the Oman Ministry of Health (MoH). The authority currently follows a notification-based regulatory system.
Does Oman follow EU MDR or US FDA regulations?
Oman does not have a standalone medical device regulation like EU MDR or US FDA 21 CFR. However, Oman recognizes approvals from major regulatory bodies, including:
US FDA (510(k), PMA)
EU CE Marking (MDR)
Health Canada
Australia TGA
Japan MHLW
What is the medical device registration process in Oman?
The medical device registration process in Oman involves:
Preparing regulatory documentation
Appointing a local Authorized Representative
Submitting a notification to DGPA&DC
Receiving market authorization
Once approved, the device is listed in the Oman medical device registry.
How long does medical device registration in Oman take?
The approval timeline is typically 3–4 months, depending on documentation quality, device complexity, and response time to DGPA&DC queries.