Clean Room Design Consultant in Oman
Expert Clean Room Solutions for Medical Device Manufacturing
At Operon Strategist, we are your trusted Clean Room Design Consultant in Oman, delivering customized solutions for medical device manufacturers. Our expert consultants design and implement controlled environments aligned with ISO 13485, ISO 14644, GMP, and Oman Ministry of Health standards to ensure sterile and compliant manufacturing.
What is a Clean Room?
A clean room is a controlled environment that limits contaminants such as dust, airborne microbes, aerosol particles, and chemical vapors. These are critical in medical device manufacturing, especially for Class II and Class III devices, where product sterility and patient safety are essential.
As your clean room design consultant, we help you build classified areas that ensure precision, safety, and regulatory alignment.

Why Clean Rooms Are Essential in Oman’s Medical Device Industry
Medical device companies in Oman must meet global quality standards and local regulations. Our clean room design consultancy ensures:
Regulatory Compliance: Adheres to ISO 13485, ISO 14644, GMP, and Ministry of Health requirements.
Product Quality & Sterility: Supports the safe production of implantable and high-risk devices.
Environmental Control: Manages particulate levels, pressure differentials, humidity, and airflow.
Efficient Layout Planning: Designs that minimize cross-contamination risks and maximize workflow efficiency.
Cost-Effective Solutions: Smart planning to stay within project budgets while achieving full compliance.
Let’s Connect! Your Queries, Our Expertise!
Clean Room Regulations We Follow
As a leading clean room design consultant, we ensure full compliance with:
- ISO 13485 – Quality Management Systems for medical devices.
- ISO 14644 – Standards for air cleanliness, airflow, and classification.
- GMP Guidelines – Layout, maintenance, sanitation, and validation protocols.
Our Clean Room Design Consulting Services
We provide end-to-end clean room design services in Oman tailored to your manufacturing needs:
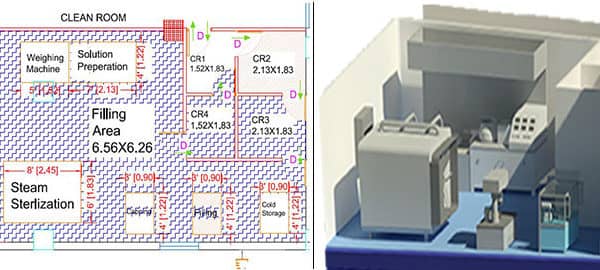
1. AutoCAD Layout & Facility Design
Custom floor plans for production, assembly, gowning, and packaging areas with detailed 2D/3D CAD drawings.
2. HVAC & Air Handling System Planning
Designs for airflow control, pressure gradients, and HEPA filtration systems to maintain air quality.
3. QMS Documentation & Validation Support
We prepare clean room documentation for ISO 13485, IQ/OQ/PQ, and risk-based validation.
4. Turnkey Clean Room Project Execution
From concept to commissioning — including HVAC installation, modular clean room construction, validation, and staff training.
Our Proven Process for Clean Room Setup
As your full-service clean room design consultant, we follow a proven, regulatory-compliant process:
- Initial Assessment: Site and product evaluation
- Design Development: Customized AutoCAD layout & airflow plan
- Regulatory Review: Ensuring ISO/GMP-compliant documentation
- Construction & Commissioning: Supervision, equipment setup, and cleanroom build
- Validation: Performance testing (IQ/OQ/PQ), pressure balancing, HEPA leak testing, and classification
- Ongoing Support: Maintenance SOPs, audit support, and environmental monitoring plans
Ready to Start Your Clean Room Project in Oman?
Why Choose Operon Strategist?
With over a decade of experience, Operon Strategist is a preferred clean room design consultant in Oman for manufacturers seeking quality, compliance, and operational efficiency. We go beyond cleanroom design by offering complete regulatory and turnkey consulting support for medical device and pharmaceutical projects.
Our Strengths:
- Regulatory expertise in ISO 13485, ISO 14644 & GMP
- Custom HVAC and cleanroom layouts for sterile manufacturing zones
- Expertise in gowning areas, airlocks, pass boxes, and pressure differentials
- Complete validation & documentation support (URS, DQ, IQ, OQ, PQ)
- End-to-end project execution with on-time delivery
- Integrated services including Medical Device Registration in Oman, CE Marking, US FDA 510(k) consulting, ISO 13485 implementation, feasibility studies, and manufacturing plant setup
By combining engineering excellence with regulatory strategy, Operon Strategist ensures your facility is audit-ready, compliant, and built for scalable growth.
FAQs
What is a clean room and why is it important for medical device manufacturing?
A clean room is a controlled environment that limits airborne particles, temperature, humidity, and contamination risks. It is critical in medical device manufacturing to ensure product safety, sterility, and regulatory compliance.
Are clean rooms mandatory for all medical device manufacturers in Oman?
Clean rooms are essential for manufacturers producing sterile or high-risk medical devices. Regulatory bodies, including Oman’s Ministry of Health, require controlled environments aligned with ISO 13485 and ISO 14644 for product approval.
How can Operon Strategist help with clean room setup in Oman?
Operon Strategist offers complete clean room design services including layout planning (AutoCAD), HVAC design, regulatory guidance, ISO 14644 validation, and turnkey project execution—from concept to compliance.
How long does it take to design and validate a clean room?
The timeline varies depending on the size and complexity of the facility, but typically ranges from 4 to 12 weeks, including design, construction oversight, and validation.
Do you help with clean room validation and documentation?
Yes, we provide full validation services including Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ) documentation, aligned with ISO and GMP requirements.