Medical Device Manufacturing in Oman: Expert Consulting Services
Optimized Medical Device Facility Layout & Compliance Consulting in Oman
Designing a compliant and efficient medical device manufacturing facility in Oman is essential for meeting regulatory requirements and ensuring smooth operations. Operon Strategist offers expert consulting services to help manufacturers plan optimized facility layouts that align with Oman’s Ministry of Health regulations and international standards such as ISO 13485, US FDA, CE Marking, and CDSCO. Our layout designs focus on streamlined workflows, contamination control, cleanroom zoning, scalability, and audit readiness. Whether you’re setting up a new plant or upgrading an existing one, we ensure your facility supports regulatory compliance, quality manufacturing, and long-term operational success.
Medical Device Manufacturing in Oman:
A medical device is a crucial instrument or apparatus designed to diagnose, monitor, or treat health conditions. Medical devices range widely in complexity and application, from basic items like tongue depressors and disposable gloves to advanced equipment such as diagnostic computers, implants, and prostheses. The development and manufacturing of these devices form an essential part of biomedical engineering, as each device must meet strict regulatory standards to ensure safe and effective use.
Purpose and Applications of Medical Devices
Medical devices serve various vital functions, including:
- Diagnosis and Treatment: Detect and treat diseases, injuries, or disabilities.
- Monitoring and Prevention: Track health conditions or prevent disease progression.
- Anatomical and Functional Support: Aid or replace anatomical structures or physiological processes.
- Reproductive Health: Certain devices control conception without relying on pharmacological means.
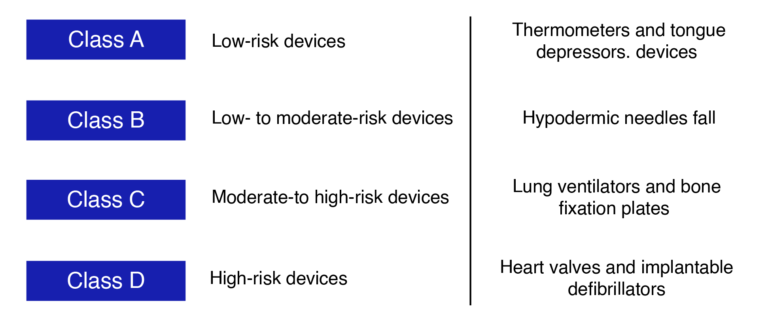
These devices are categorized into different classes by regulatory authorities worldwide based on factors such as complexity, use, and risk potential.
Medical Device Manufacturing in Oman
Oman’s healthcare sector has grown significantly, making it a burgeoning hub for the medical device industry. Manufacturers interested in entering the Omani market must adhere to the country’s specific laws and regulations governing medical device registration. Familiarity with these regulatory processes is crucial for smooth market entry and compliance.
Let’s Connect! Your Queries, Our Expertise
Medical Device Classifications and Regulatory Standards
Medical devices are categorized based on design complexity and intended use, with distinct regulations for each class to ensure safety and efficacy. Regulatory authorities also consider combination products that integrate medical devices with pharmaceutical components. Ensuring compliance with these classifications is a necessary step for manufacturers aiming to launch products in diverse global markets, including Oman.
Connect with us today to turn your medical device project into a regulatory success!
Need a Medical Device Manufacturing Consultant?
Planning to set up or expand your medical device manufacturing facility in Oman? Our expert consultants at Operon Strategist are here to guide you through every step from regulatory compliance and facility design to market readiness. With deep knowledge of Oman’s Ministry of Health requirements and global standards like ISO 13485, USFDA, and CE Marking, we help you simplify complex regulations and accelerate your project with confidence.
FAQs
Why is proper facility layout design important in medical device manufacturing?
A well-planned facility layout is essential for ensuring compliance with regulatory bodies such as Oman’s Ministry of Health (MoH), ISO 13485, US FDA, CDSCO, and CE Marking requirements. It minimizes contamination risks, improves operational efficiency, ensures product safety, and facilitates successful audits and certifications.
What are the key regulatory standards for setting up a medical device manufacturing facility in Oman?
Manufacturers in Oman must comply with local regulations issued by the Oman MoH and align with globally accepted standards including ISO 13485, CE Marking under EU MDR, FDA 21 CFR Part 820, and SFDA for exporting to the Gulf and international markets. These standards help ensure safe, effective, and high-quality manufacturing processes.
What services does Operon Strategist offer for medical device facility setup in Oman?
Operon Strategist provides comprehensive turnkey consulting solutions for medical device facility setup in Oman. Services include:
Facility layout and cleanroom design
Regulatory strategy and market entry planning
ISO 13485 QMS implementation
Equipment sourcing and validation
Cleanroom classification as per ISO 14644
Complete project management support until commissioning
How does a compliant facility layout reduce production delays and regulatory risks?
A compliant layout ensures efficient movement of materials and personnel, proper zoning (sterile and non-sterile), and built-in quality checkpoints. This reduces operational errors, enhances production flow, minimizes contamination risks, and ensures readiness for regulatory inspections—thereby preventing costly delays, non-conformities, or product recalls.
Can Operon Strategist help Omani manufacturers export medical devices globally?
Yes. Operon Strategist designs facilities and regulatory pathways to meet CE, US FDA, and SFDA requirements. We help Omani manufacturers develop compliant technical documentation, quality systems, and facility designs to enable smooth export of medical devices to markets in Europe, the USA, and the GCC region.