Medical Device Validation & Documentation Services in Oman
What is Medical Device Validation?
Medical Device Process Validation is a process of establishing documentary evidence demonstrating that a procedure, process or activity carried out in production maintains the desired level of compliance at all stages. In straightforward words, the interaction approval for a medical device is the assortment and assessment of information, from the cycle configuration stage till the creation, as it gives logical proof that the cycle is able to do reliably giving quality items. In simple words, the process validation medical device is the collection and evaluation of data, from the process design stage till the production, as it gives scientific evidence that the process is capable of consistently providing quality products.
Let's Connect! Your Queries, Our Expertise!
What is the Difference Between Process Verification and Validation?
According to USFDA process validation means establishing the objective evidence that a process consistently meets the products Predetermined specifications. Verification process means verifying the quality of product but testing every single device is impractical, and process validation comes into picture. The process validation completes the quality assurance need. As an ISO 13485 medical device consultant We know the guidelines provided by the regulatory authority and we help manufacturers to implement them correctly.
Medical Device Process Validation Services:
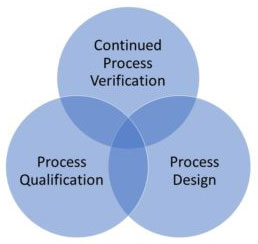
Medical Device Process Validation is Divided into the Following Sub-Sections
Medical Device Process Validation is a multifaceted process that involves various sub-sections to ensure the consistent quality and performance of medical devices. These sub-sections encompass critical aspects such as equipment validation, personnel validation, facility validation, and analytical method validation. Each of these components plays a vital role in establishing the reliability and safety of medical device manufacturing processes, contributing to the overall effectiveness of the validation process. Following are sub-sections of process validation:
- HVAC validation
- Equipment validation
- Process validation
- Facilities validation
- Cleaning validation
- Analytical method validation
- Personnel validation
- Packaging validation
- Computer system validation
Ensure Regulatory-Ready Validation with Expert Support!
Why choose Operon Strategist as Validation Process Consultant?
At Operon Strategist, we offer comprehensive medical device validation consulting services in Oman, helping manufacturers meet both local and international regulatory standards. Our team of validation experts provides guidance on design qualification, site acceptance testing, and the preparation of FDA-compliant process validation protocols and reports. We support facility and utility qualification, including HVAC, WFI, and compressed air systems, as well as manufacturing and packaging process validation and design validation. With global experience and a strong client base in Oman, we deliver tailored solutions that ensure compliance with ISO 13485, US FDA, CE Marking, and Oman MoH requirements.
FAQs
What is process validation in medical device manufacturing?
Process validation is a documented procedure that ensures manufacturing processes consistently produce medical devices that meet pre-defined specifications and quality standards.
Why is validation important for medical device manufacturers in Oman?
Validation ensures compliance with Oman MoH regulations and global standards like FDA 21 CFR Part 820, ISO 13485, and CE Marking. It minimizes risk, supports product safety, and improves audit outcomes.
How does Operon Strategist support validation for medical devices in Oman?
Operon Strategist offers complete validation consulting services for medical device manufacturers, including documentation development, protocol execution, testing support, and regulatory guidance specific to Oman and the GCC market.
How long does it take to complete validation documentation for medical devices?
Depending on the device type and regulatory scope, the process may take 4 to 12 weeks. Operon Strategist accelerates this timeline with expert project management and ready-to-use validation templates.
Can Operon Strategist assist with validation documentation for export from Oman?
Yes, Operon Strategist helps Oman-based manufacturers develop validation documentation compliant with international markets such as the EU, USA, and Saudi Arabia, enabling seamless global exports.